Method for compounding 2,6-dichloropurine nucleoside by using inosine as raw material
A technology of dichloropurine nucleoside and chlorotriacetyl purine nucleoside, which is applied in the field of synthesizing 2,6-dichloropurine nucleoside, can solve the problems of many synthesis steps, easy residue, high price, etc., and achieve simple process and total yield The effect of high efficiency and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
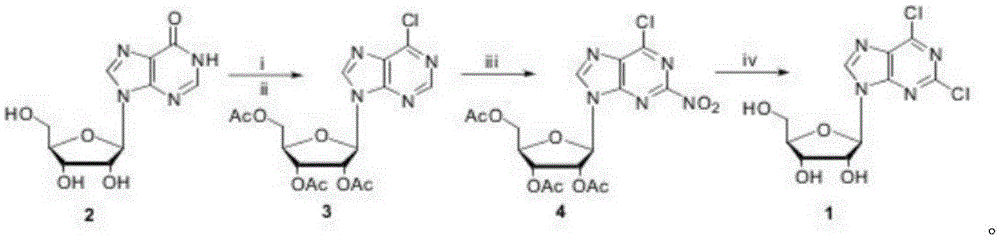
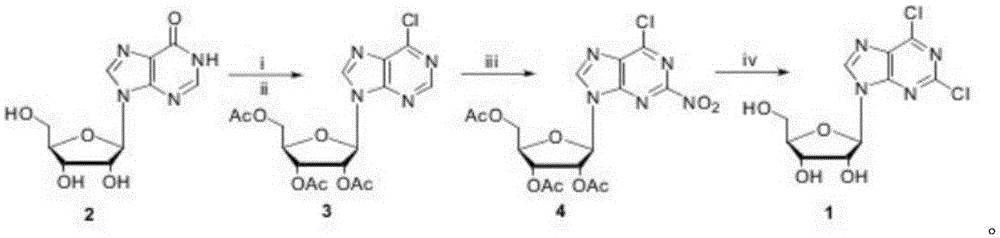
[0018] A method for synthesizing 2,6-dichloropurine nucleoside with inosine as raw material is characterized in that, using cheap inosine as raw material, 6-chlorotriacetyl purine nucleoside is obtained; then carry out nitration reaction, in 2 2-nitro-6-chlorotriacetylpurine nucleoside is obtained by introducing a nitro group at the position; finally, in an ethanol solution saturated with hydrogen chloride gas, the two-step reaction of removing acetyl group and nitrochlorination is completed to obtain 2,6-diacetyl purine nucleoside Chloropurine nucleosides.
Embodiment 2
[0020] The synthetic route of the present invention is as follows:
[0021]
[0022] in
[0023] Synthesis of 6-Chlorotriacetylpurine Nucleoside (3)
[0024] According to the literature (Zhou Le, Shi Yuangang, Wang Jianchen. Synthesis of 6-chloro-9-β-D-ribofuranosylpurine. Chemical Bulletin, 1996,5:31-32.), the product 1 The HNMR data are consistent with the literature reports.
[0025] Synthesis of 2-nitro-6-chlorotriacetylpurine nucleoside (4)
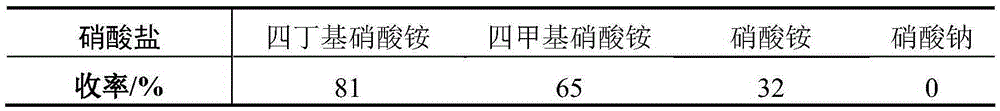
[0026] Tetrabutylammonium nitrate (10.1g, 33.0mmol) was dissolved in anhydrous dichloromethane (50mL), cooled to 0°C, added trifluoroacetic anhydride (4.6mL, 33.0mmol), stirred for 20 minutes, added freshly prepared 6-Chlorotriacetylpurine nucleoside (3, 9.1g, 22mmol), kept at 0°C, reacted for 5 hours, added saturated sodium bicarbonate solution (100mL), stirred, separated the organic phase, and used anhydrous dichloro Methane (20 mL) was extracted twice, the organic phase was collected, dried, and the solvent was removed unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com