Novel synthesis of nucleoside 5'-triphosphates and their derivatives
A technology for triphosphoric acid and derivatives, which is applied in the preparation of sugar derivatives, sugar derivatives, and sugar derivatives, and can solve the problems of difficult purification and separation of desired products, low yields, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
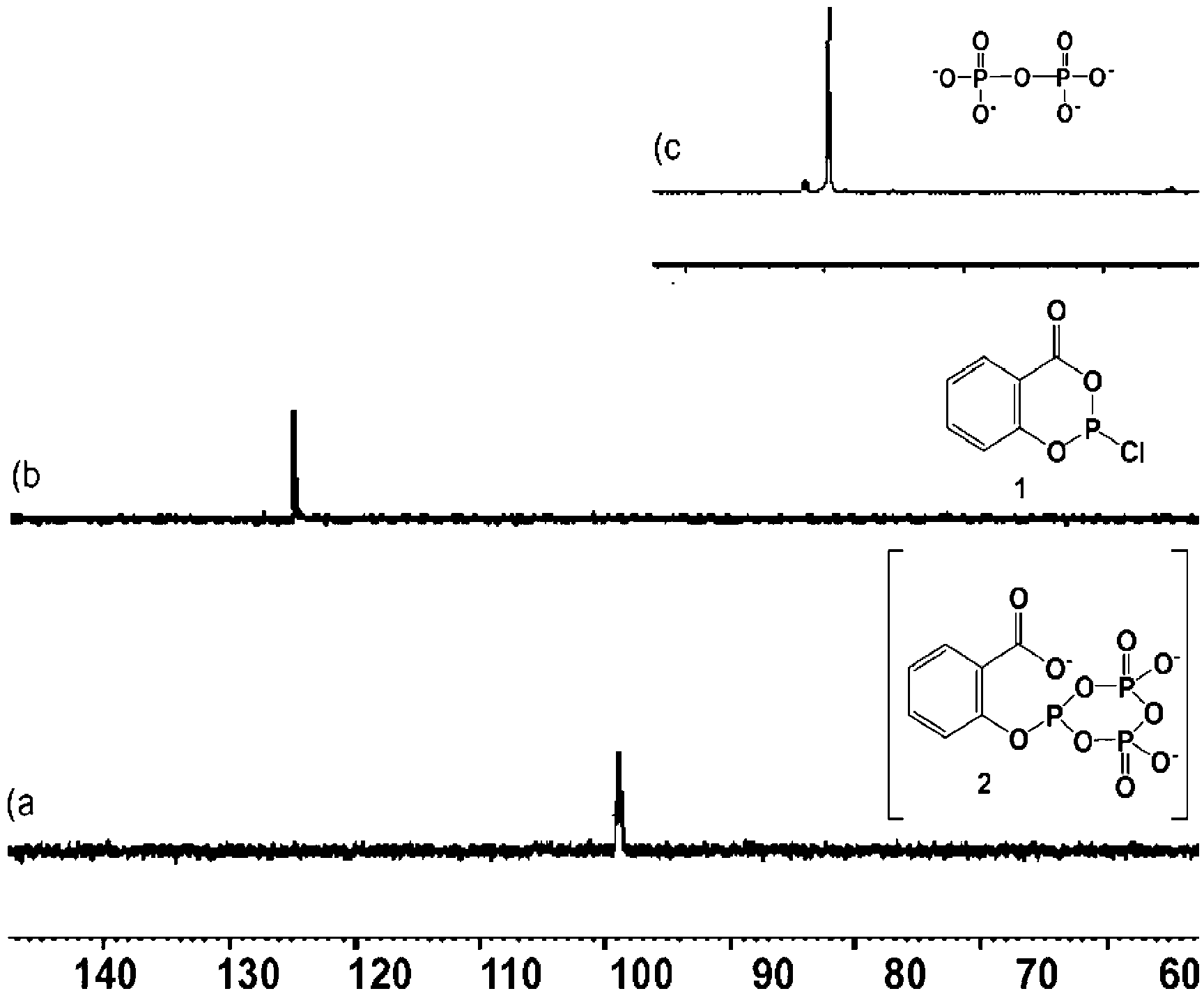
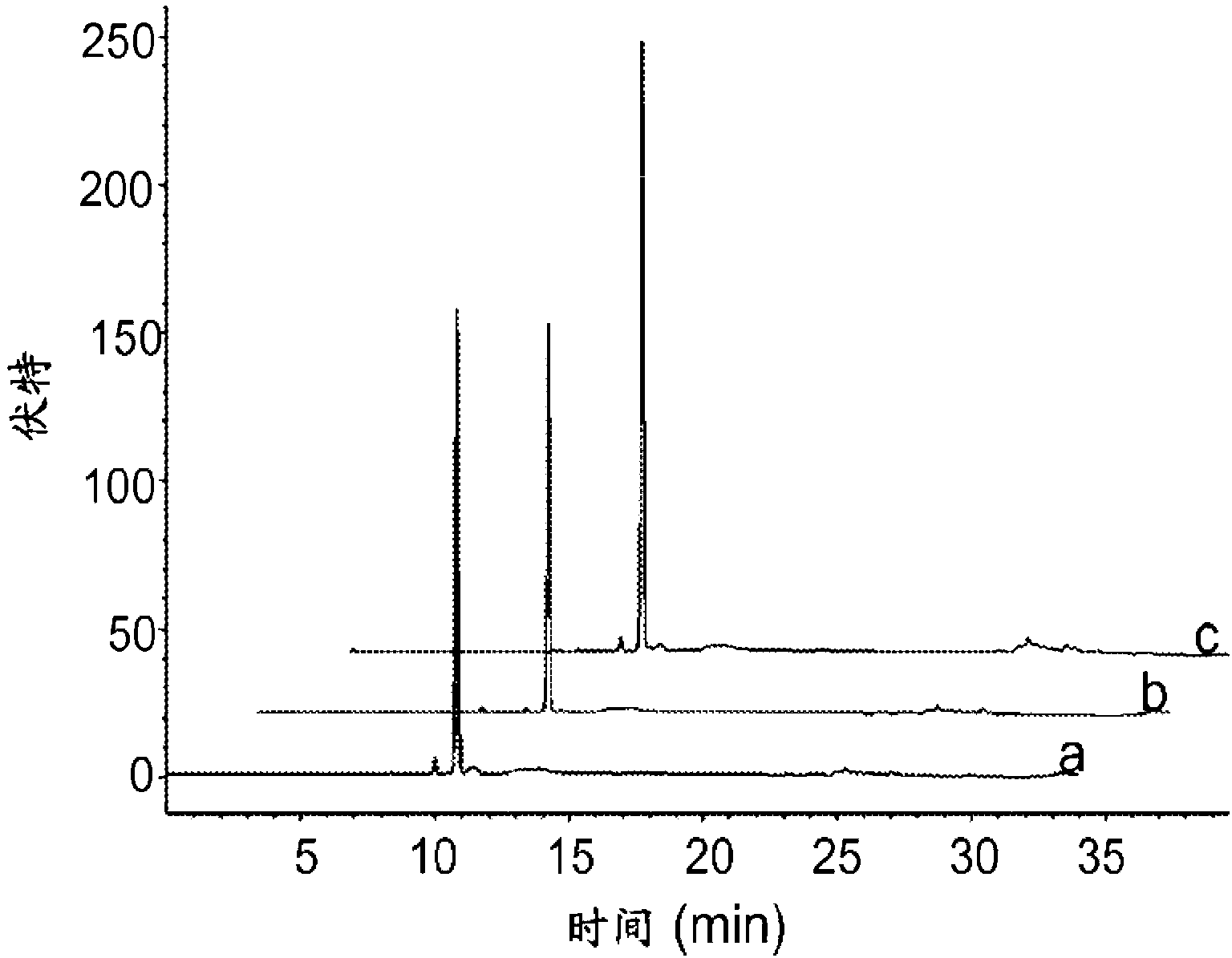
[0062] The crude yield of natural 2'-deoxynucleoside-5'-triphosphates (e.g., nucleosides of dA, dC, dG, or T) is at least greater than about 80% prior to any optimization of the preparation methods disclosed above; And the HPLC yields of natural 2'-deoxynucleoside-5'-triphosphates (e.g., nucleosides of dA, dC, dG, or T) after separation (e.g., NaCl-ethanol separation) range from about 19% to about 46% range. After optimizing the reaction conditions, the isolated yields ranged from about 80% to about 100%. To mitigate the effect of the undesired 3'-triphosphate, it must be ensured that the starting reagent is completely consumed prior to addition of the nucleoside to form intermediate 2. No difficulties were encountered during the HPLC purification of nucleoside triphosphates.
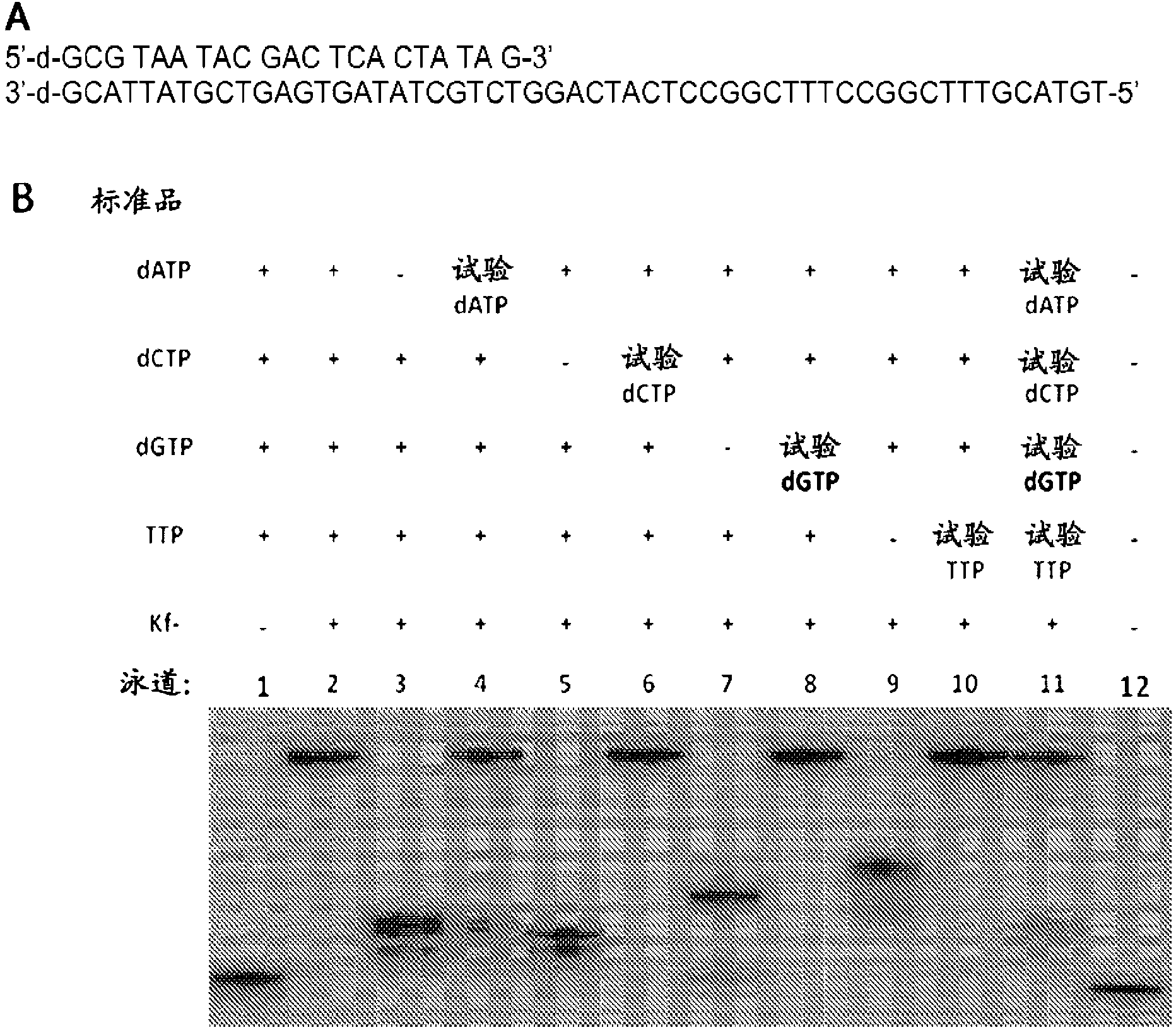
[0063]In some other forms, the disclosed methods are those wherein the method can be used to synthesize alpha-modified nucleoside 5'-triphosphates. In some other forms, the disclosed methods are thos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com