Gene base editor
A technology of editing and residues, applied in chemical instruments and methods, antibody mimics/scaffolds, hybrid peptides, etc., can solve the problem of ineffective correction, limited effective editing sites of base editors, and deletion of RNA splicing sites And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
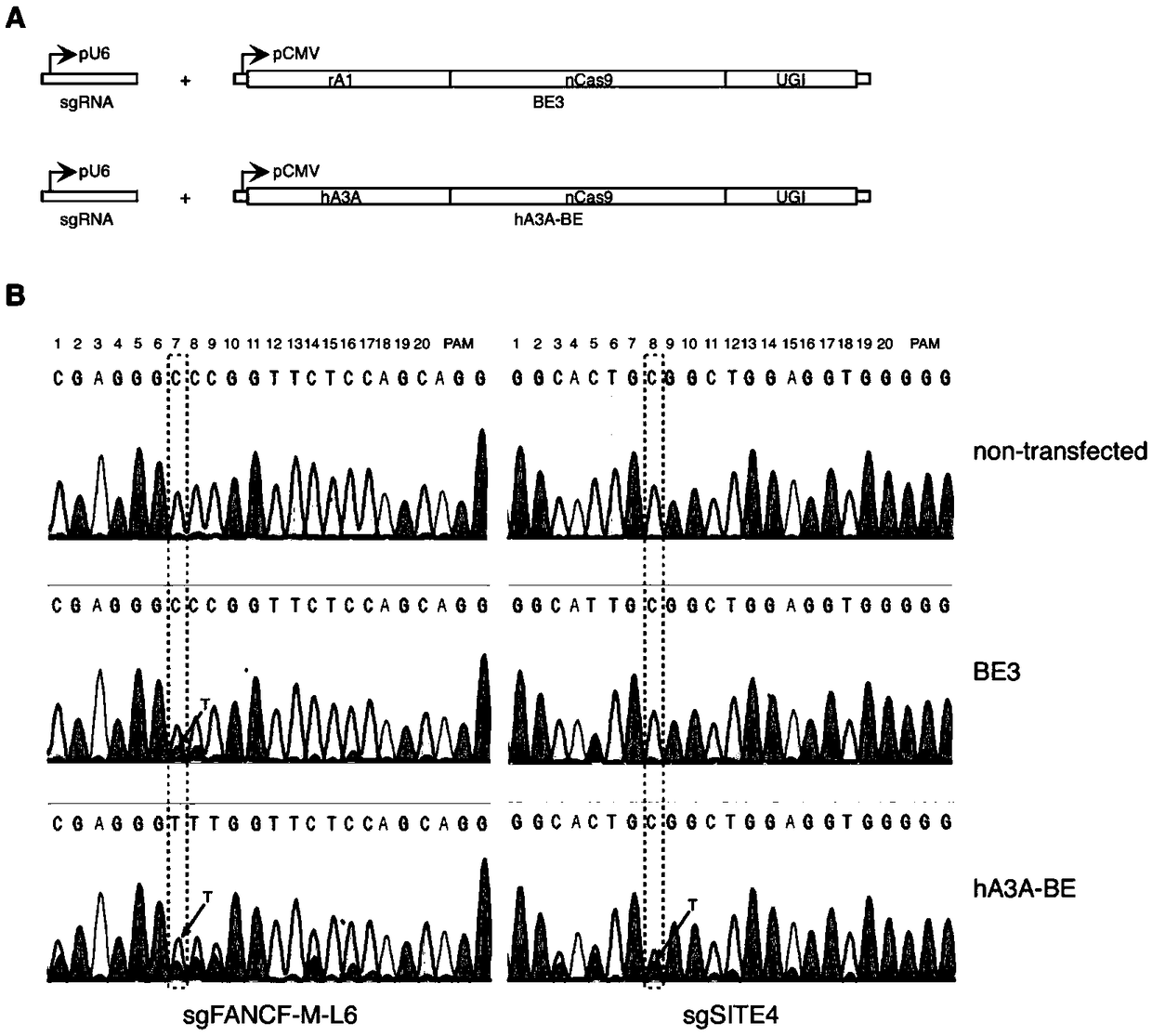
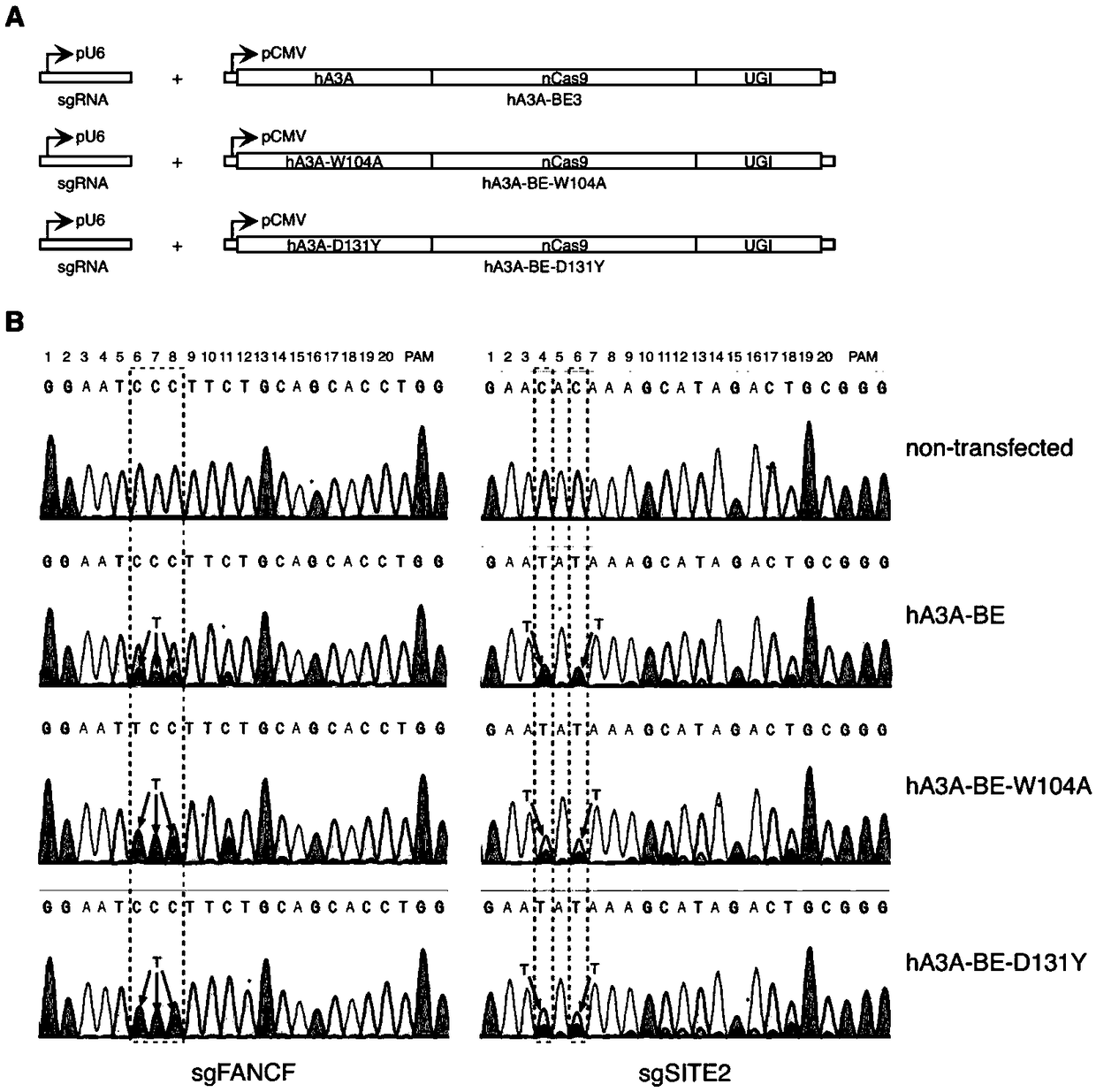
[0102] Example 1 The FANCF site of the human genome uses the hA3A-BE base editor to realize efficient base editing of cytosine at the GpC site
[0103] A gene base editor, including hA3A-BE expression vector and sgRNA expression vector. The sequence of the hA3A-BE expression vector is SEQ ID NO:16.
[0104] The preparation and base editing method of the above-mentioned base editing system are as follows:
[0105] 1.1 Experimental materials
[0106] 1.1.1 Reagents and plasmids
[0107] Primers were synthesized from Suzhou Jinweizhi Biological Co., Ltd.; restriction enzymes, DNA ligase, high-fidelity DNA polymerase Purchased from NEB Company; Plasmid Recombination Kit Clone Purchased from Vazyme; pCMV-BE3 from addgene website, the article number is 73021; DNA gel recovery kit was purchased from Corning; transfection reagent LTX, Available from Thermo Fisher; QuickExtract TM Genomic DNA extraction reagents were purchased from Illumina.
[0108] 1.1.2 Cell lines
[...
Embodiment 2
[0129] Example 2 The human genome HEK293-Site4 site uses the hA3A-BE base editor to realize efficient base editing of cytosine at the GpC site
[0130] A gene base editor, including hA3A-BE expression vector and sgRNA expression vector. The sequence of the hA3A-BE expression vector is SEQ ID NO:16.
[0131] The preparation and base editing method of the above-mentioned base editing system are as follows:
[0132] 2.1 Experimental materials
[0133] 2.1.1 Reagents and plasmids: Same as Example 1.
[0134] 2.1.2 Cell lines: Same as Example 1.
[0135] 2.2 Experimental method
[0136] 2.2.1 Construction of hA3A-BE expression vector: same as Example 1.
[0137] 2.2.2 Construction of sgRNA expression plasmid
[0138] Anneal the following primers 28 and 29, and connect the annealed product into the sgRNA expression vector pGL3-u6-sgRNA-puro digested with the restriction endonuclease BsaI to obtain the sgRNA expression plasmid targeting the HEK293-Site4 site of the human genome...
Embodiment 3
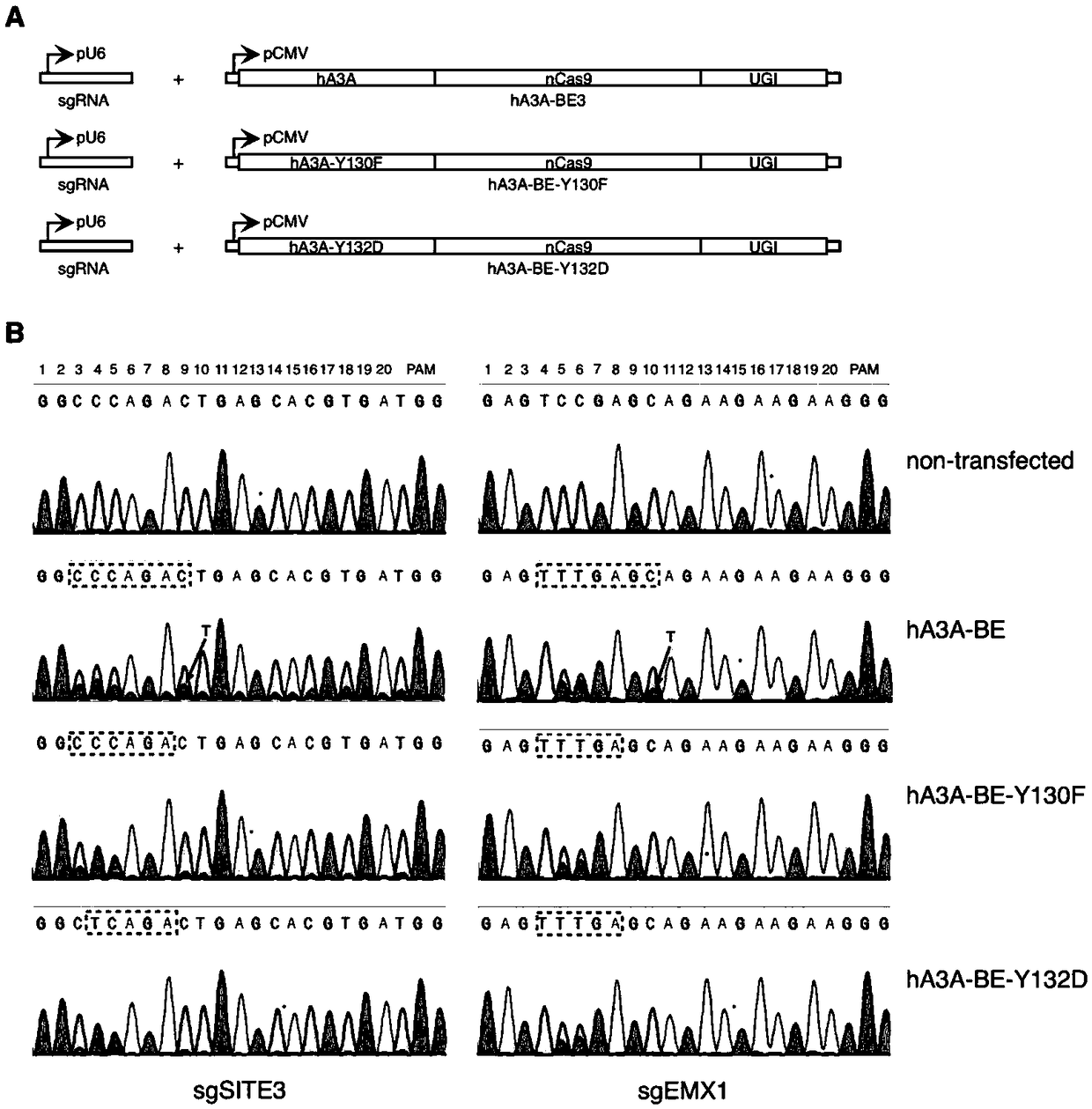
[0153] Example 3 The human genome HEK293-Site3 site uses the hA3A-BE-muts base editor to achieve high-precision and high-efficiency base editing
[0154] A gene base editor, including hA3A-BE expression vector, hA3A-BE-Y130F expression vector, hA3A-BE-Y132D expression vector and sgRNA expression vector. The sequence of the hA3A-BE expression vector is SEQ ID NO: 16; the sequence of the hA3A-BE-Y130F expression vector is SEQ ID NO: 17; the sequence of the hA3A-BE-Y132D expression vector is SEQ ID NO: 18.
[0155] The preparation and base editing method of the above-mentioned base editing system are as follows:
[0156] 3.1 Experimental materials
[0157] 3.1.1 Reagents and plasmids: Same as Example 1.
[0158] 3.1.2 Cell lines: Same as Example 1.
[0159] 3.2 Experimental method
[0160] 3.2.1 Construction of hA3A-BE expression vector: same as Example 1.
[0161] 3.2.2 Construction of hA3A-BE-Y130F expression vector
[0162] Using hA3A-BE as a template, PCR was performed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com