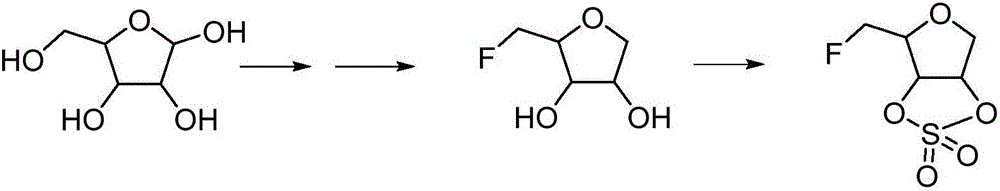
Synthesis process of fluoronucleoside
A synthesis process and technology for nucleoside substitution, which is applied in the field of synthesis process of nucleoside derivatives, can solve the problems of increased equipment requirements, low operating safety, increased energy consumption, etc., to reduce equipment requirements, improve safety, reduce The effect of energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] This embodiment relates to a synthesis process of fluorinated nucleosides, which consists of the following steps:
[0020] Step 1, reflux cytosine and hexamethyldisilazane under the catalysis of ammonium sulfate for 2 hours, the molar ratio of cytosine, hexamethyldisilazane and ammonium sulfate is 1:2:1, and then add Appropriate amount of isopropanol, and heat up to 60°C to obtain cytosine silyl ether protecting group solution;
[0021] Step 2, dissolve 2-deoxy-2,2-difluoro-D-erythro-pentafuranose-3,5-diphenylmethyl ester-1-methanesulfonate in an appropriate amount of isoamyl alcohol and stir, then add The catalyst phosphotungstic acid, the weight ratio of 2-deoxy-2,2-difluoro-D-erythro-pentafuranose-3,5-diphenylmethyl ester-1-methanesulfonate to the catalyst is 100:1.5, And heated to 73°C to obtain 2-deoxy-2,2-difluoro-D-erythro-pentofuranose-3,5-diphenylmethyl ester-1-methanesulfonate solution;
[0022] Step 3, add hydroxyapatite powder to the cytosine silyl ether p...
Embodiment 2
[0025] This embodiment relates to a synthesis process of fluorinated nucleosides, which consists of the following steps:
[0026] Step 1, reflux cytosine and hexamethyldisilazane under the catalysis of ammonium sulfate for 2 hours, the molar ratio of cytosine, hexamethyldisilazane and ammonium sulfate is 1:2:1, and then add Appropriate amount of isopropanol, and the temperature was raised to 65°C to obtain cytosine silyl ether protecting group solution;
[0027] Step 2, dissolve 2-deoxy-2,2-difluoro-D-erythro-pentafuranose-3,5-diphenylmethyl ester-1-methanesulfonate in an appropriate amount of isoamyl alcohol and stir, then add Catalyst phosphomolybdic acid, the weight ratio of 2-deoxy-2,2-difluoro-D-erythro-pentafuranose-3,5-diphenylmethyl ester-1-methanesulfonate to the catalyst is 100:1.5, And heated to 75°C to obtain 2-deoxy-2,2-difluoro-D-erythro-pentafuranose-3,5-diphenylmethyl ester-1-methanesulfonate solution;
[0028] Step 3, adding hydroxyapatite powder to the cyto...
Embodiment 3
[0031] This embodiment relates to a synthesis process of fluorinated nucleosides, which consists of the following steps:
[0032] Step 1, reflux cytosine and hexamethyldisilazane under the catalysis of ammonium sulfate for 3h, the molar ratio of cytosine, hexamethyldisilazane and ammonium sulfate is 1:2:1, and then add Appropriate amount of isopropanol, and the temperature was raised to 70°C to obtain cytosine silyl ether protecting group solution;
[0033] Step 2, dissolve 2-deoxy-2,2-difluoro-D-erythro-pentafuranose-3,5-diphenylmethyl ester-1-methanesulfonate in an appropriate amount of isoamyl alcohol and stir, then add The catalyst phosphotungstic acid, the weight ratio of 2-deoxy-2,2-difluoro-D-erythro-pentafuranose-3,5-diphenylmethyl ester-1-methanesulfonate to the catalyst is 100:1.5, And heated to 70°C to obtain 2-deoxy-2,2-difluoro-D-erythro-pentafuranose-3,5-diphenylmethyl ester-1-methanesulfonate solution;
[0034]Step 3, add hydroxyapatite powder to the cytosine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com