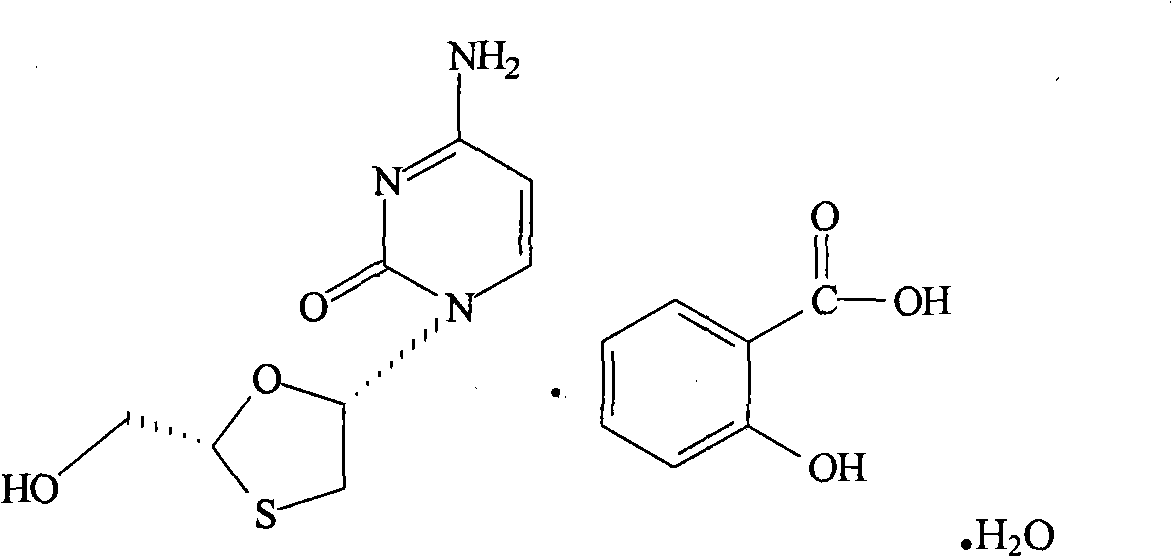
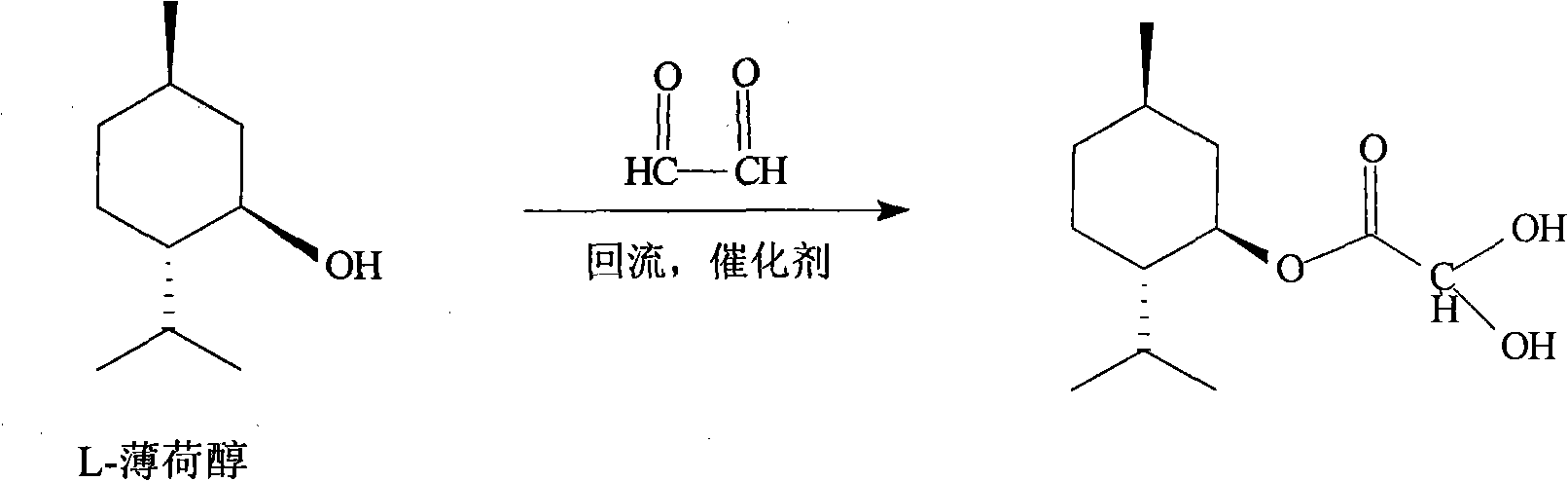
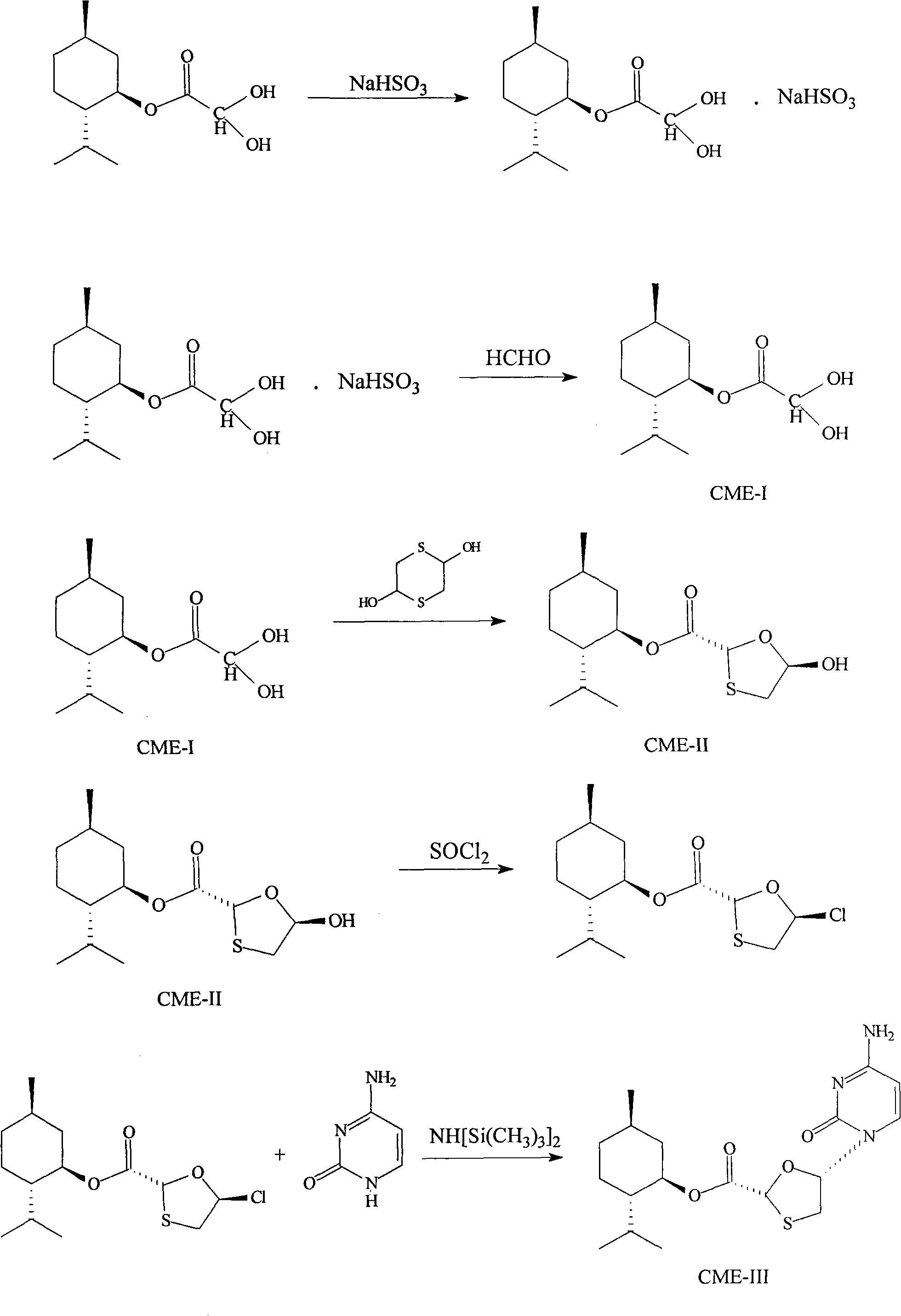
Synthesis and preparation process of lamivudine intermediate HDMS
A preparation process, lamivudine technology, is applied in the field of synthesis and preparation process of lamivudine intermediate HDMS, which can solve the problems of cost reduction, inconvenient operation, low yield, etc., and achieve cost reduction, stable product quality, Effect of Yield Improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0040] 1. Preparation of menthyl glyoxylate CME-I:
[0041] Add 250ml of cyclohexane, 260g (1.6667mol) of L-menthol, 42g (0.5676mol) of glyoxylic acid, and 1.5g of concentrated sulfuric acid into a 500ml four-necked bottle, heat up to reflux, and react under reflux for 6 hours. Analyzing the end point of the reaction, CME-I: menthol is about 50: 50, and the water in the reaction is taken out during reflux. After the reaction, the temperature was lowered to 35° C., and the organic layer was washed twice with 2×100 ml of purified water. The organic layer is set aside.
[0042] Add 1000ml of purified water and 50g (0.4807mol) of sodium bisulfite to another 2000ml four-necked bottle, and stir until clarified. Add the above organic layer into the sodium bisulfite solution, and stir at 20-30° C. for 12 hours. The reaction liquid is analyzed by gas chromatography at the end of the reaction, and the CMI-I content is ≤10%. After the reaction was completed, the layers were separated,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com