Preparation method of lamivudine and intermediate thereof
A technology for lamivudine and intermediates, applied in organic chemistry, antiviral agents, etc., can solve problems such as corrosion equipment, introduction, and complicated operation, and achieve the effects of low production cost, simplified process steps, and safe and reliable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
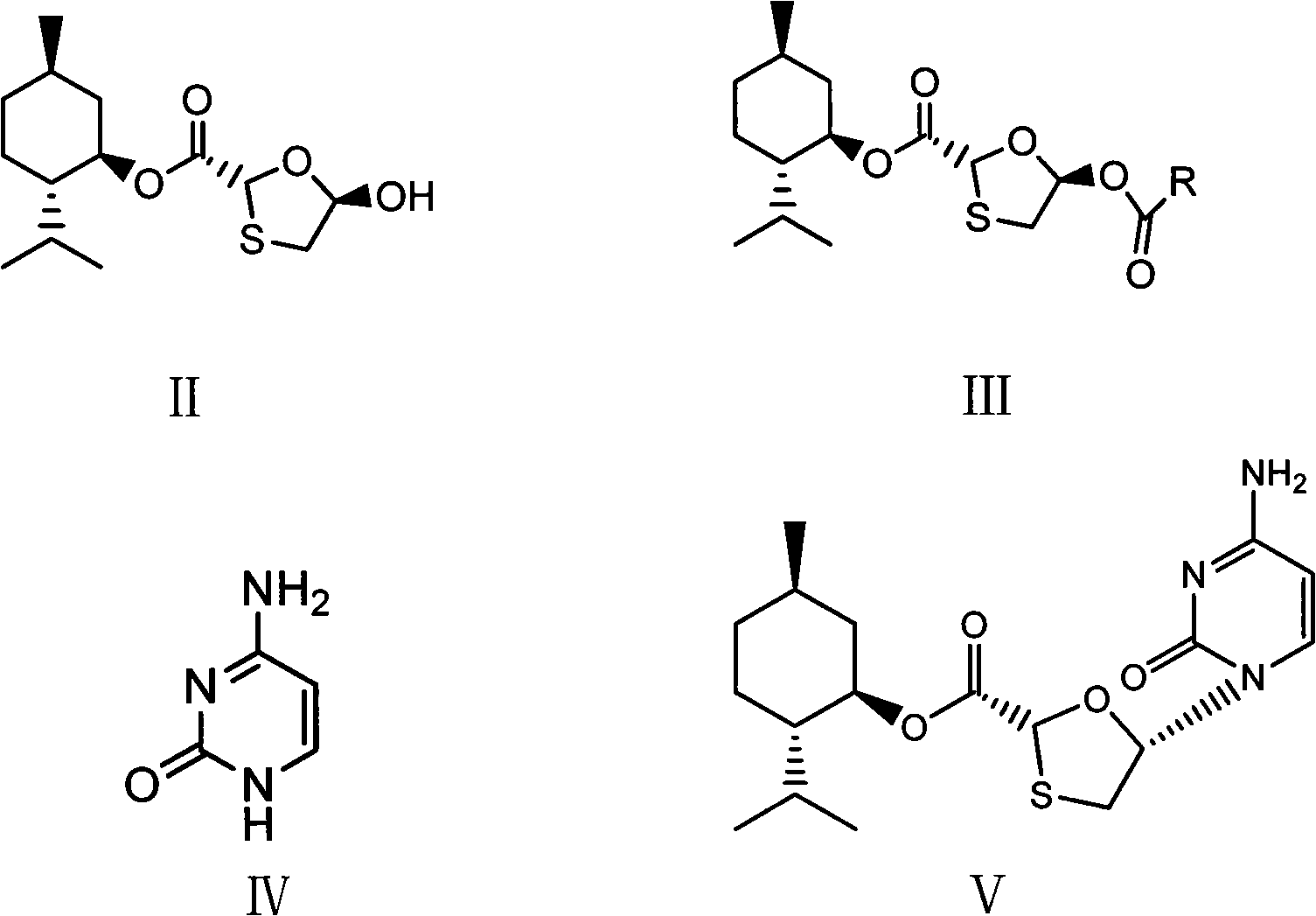
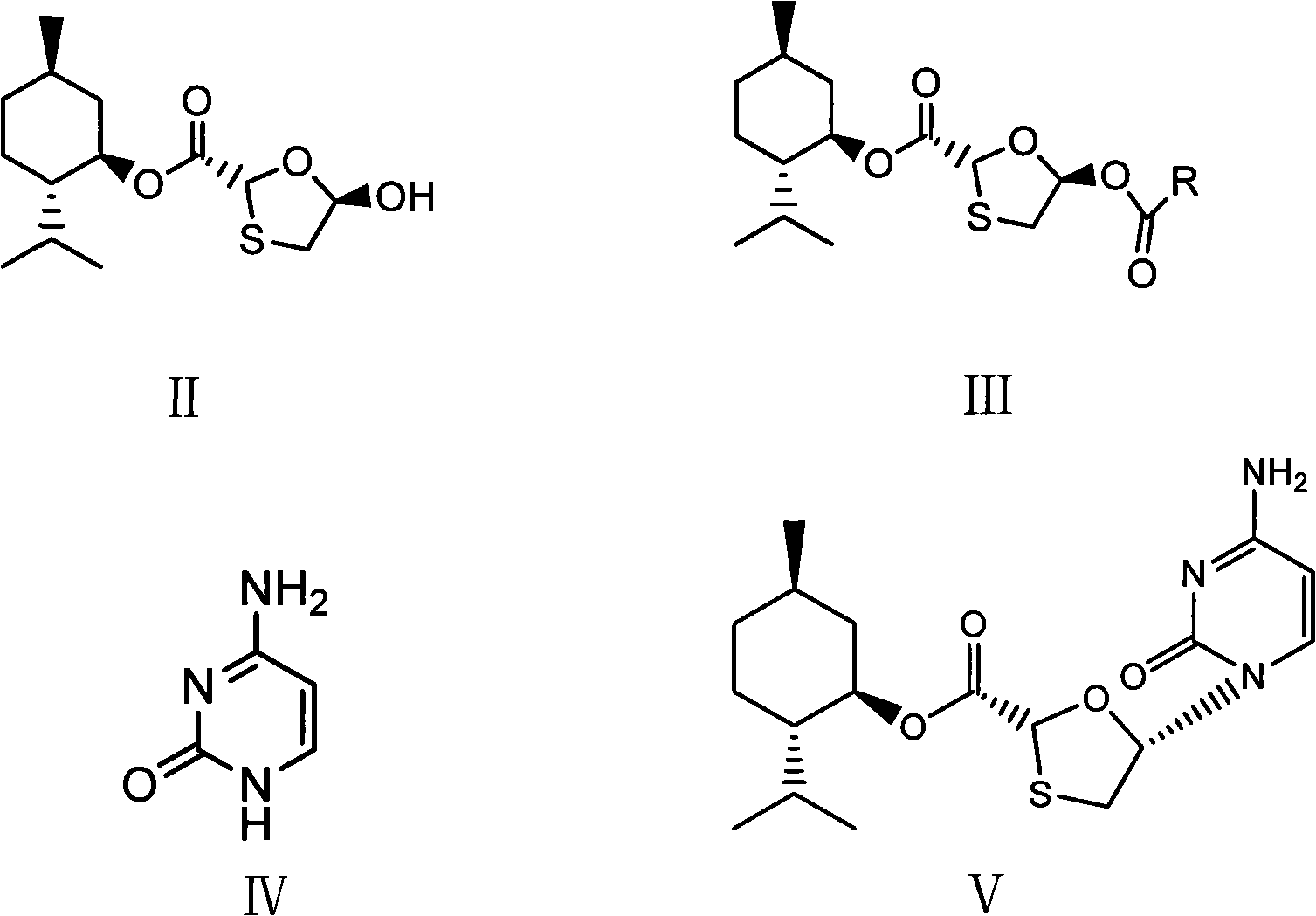
[0020] (1) Synthesis of (2R, 5R)-5-acetyl-[1,3]oxathiolane-2-carboxylic acid (2S-isopropyl-5R-methyl-1R-cyclohexyl) ester
[0021]
[0022] 28.8g (2R, 5R)-5-hydroxyl-[1,3]oxathiolane-2-carboxylic acid (2S-isopropyl-5R-methyl-1R-cyclohexyl) ester, 12.2g Acetic anhydride, 0.1g DMAP, 8.2g pyridine, and 57.6g dichloromethane were added to a 100ml reaction bottle, stirred at 40°C until the raw materials were completely reacted, the reaction solution was concentrated to dryness, and the residue was crystallized with petroleum ether to obtain 29.7g of white needle-like solid. Yield 90.0%.
[0023] (2) Synthesis of (2R, 5R)-5-acetyl-[1,3]oxathiolane-2-carboxylic acid (2S-isopropyl-5R-methyl-1R-cyclohexyl) ester
[0024]
[0025] 28.8g (2R, 5R)-5-hydroxyl-[1,3]oxathiolane-2-carboxylic acid (2S-isopropyl-5R-methyl-1R-cyclohexyl) ester, 9.4g Add acetyl chloride, 8.2g pyridine, 0.1g DMAP, and 57.6g dichloromethane into a 100ml reaction bottle, stir at 10°C until the raw materials ...
Embodiment 2
[0036] (2R,5S)-5-(4-Amino-2-oxopyridin-1-yl)-[1,3]oxathiolane-2-carboxylic acid (2S-isopropyl-5R-methyl Synthesis of 1R-cyclohexyl) ester
[0037]
[0038] (1) Heat 13.3g of cytosine, 27.9ml of hexamethylaziasilane, 0.08ml of methanesulfonic acid, and 33.0ml of toluene to reflux. After complete dissolution, nitrogen protection is used to cool down, and 33g of (2R, 5R)-5-acetyl- [1,3] Oxathiolane-2-carboxylic acid (2S-isopropyl-5R-methyl-1R-cyclohexyl) ester and 84ml methylene chloride solution, add 40g iodotrimethylsilane dropwise, After the drop is completed, lower the temperature at 35-40°C until the reaction of the raw materials is complete, then add hydrochloric acid dropwise to pH ≈ 2.5, and keep at 30-35°C. After dripping, stir for 1 hour, filter, the filter cake becomes granular, wash the filter cake with saturated sodium bicarbonate, and dry to obtain 33.5 g of white solid, yield 88.0%, melting point: 219°C (decomposition).
[0039] (2) Heat 13.3g of cytosine, 27....
Embodiment 3
[0043] Synthesis of Lamivudine
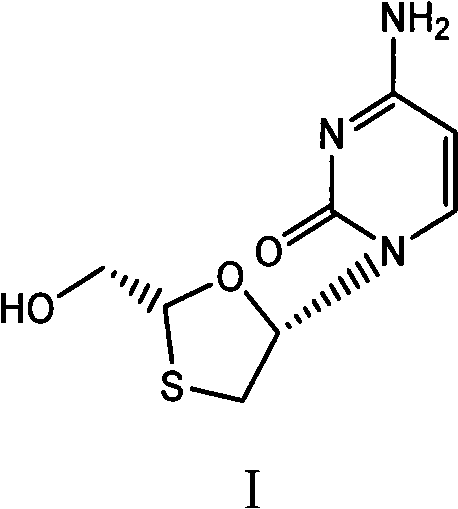
[0044] 38.1g (2R, 5S)-5-(4-amino-2-oxopyridin-1-yl)-[1,3]oxathiolane-2-carboxylic acid (2S-isopropyl-5R -Methyl-1R-cyclohexyl) ester and 250ml of absolute ethanol mixture were stirred at 15-20°C, and 7.6g of sodium borohydride was added in batches, and the temperature was kept at 15-20°C and stirred for 2 hours, and the reaction was completed by TLC monitoring, 40 Concentrate the reaction solution at ℃ to dryness, add 10 ml of water and 100 ml of n-hexane, stir for 30 minutes at 20-25 °C, separate the n-hexane, filter to obtain crude lamivudine, recrystallize from absolute ethanol to obtain lamivudine 16.1, and obtain rate 70.3%, 1 H-NMR (600MHz, DMSO): δ3.055(1H, dd), δ3.409(1H, dd), δ3.704(2H, m), δ5.174(1H, t), δ5.321(1H , t), δ5.739(1H, d), δ6.209(1H, t), δ7.244(2H, s), δ7.823(1H, d), 13 C-NMR (600MHz, DMSO): δ36.967, δ63.523, δ86.508, δ87.217, δ94.621, δ141.656, δ155.390, δ166.337.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com