Erythrocyte membrane-encapsulated tetrandrine PLGA nanoparticle and preparation method and application thereof
A technology of erythrocyte membrane and tetrandrine, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of short maintenance time of blood drug concentration, local pain or phlebitis, biological Problems such as low utilization can achieve the effects of prolonging blood half-life, delaying release, and reducing irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
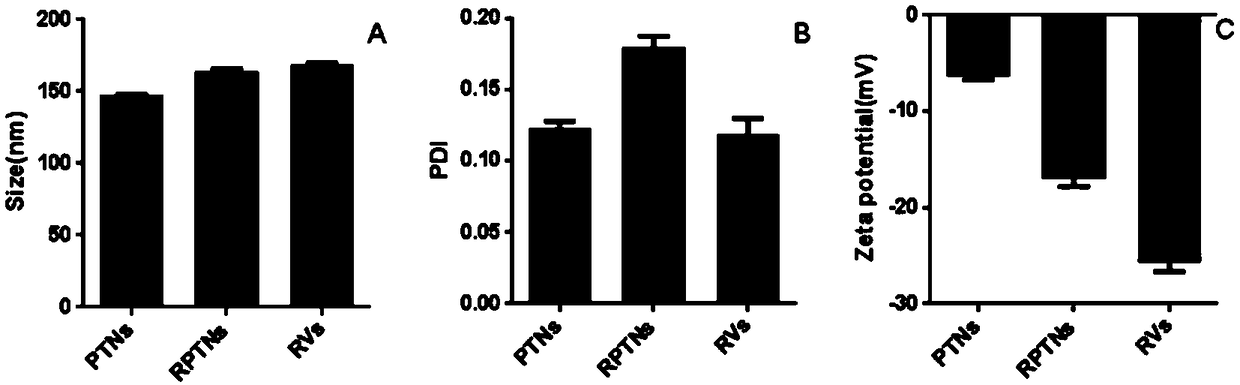
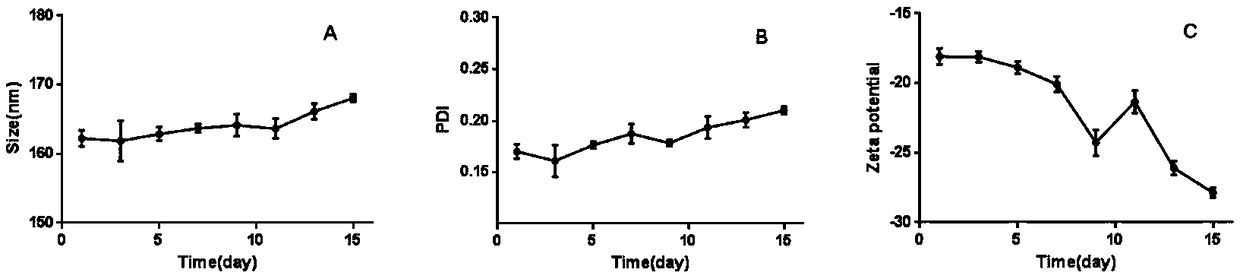
[0054] The preparation of embodiment 1 nanoemulsion (PTNs)
[0055] Weigh TET and PLGA (molecular weight: 30,000) respectively, add acetone to dissolve, so that the concentrations of TET and PLGA are 1mg / mL and 20mg / ml respectively, and then slowly add 1mL of the organic phase under the condition of magnetic stirring (300rpm) In the 20mL water phase, (the emulsifier in the water phase is poloxamer, and the emulsifier quality is 0.2g), so that the mass ratio of tetrandrine, PLGA, and emulsifier is that the mass ratio of the three is 1:20:200, The acetone was volatilized and removed by magnetic stirring at room temperature for 3 hours to obtain a nanoemulsion solution.
Embodiment 2
[0056] Example 2. Preparation of erythrocyte membrane
[0057] Take healthy rats, weigh them, anesthetize with 100g / 0.7mL intraperitoneal injection of chloral hydrate, take blood from the abdominal aorta, divide the blood into EP tubes containing 0.05mL heparin sodium, and centrifuge (3800rpm, 5min, 4°C) Remove the supernatant and white blood cell layer, add PBS solution to mix, repeat washing three times, and dilute to 1mL with PBS. Divide each tube of the mixed solution obtained at the above constant volume into four EP tubes containing 0.01mL heparin sodium, then add 0.9mL LEDTA solution (0.2mM), mix well, and lyse the red blood cells in the hypotonic medium to release hemoglobin, and then Then add 0.1mL 10 times concentration PBS and mix well, centrifuge (13200r / min, 10min, 4°C) to wash, remove the supernatant, keep the red blood cell membrane at the bottom, repeat this process more than three times until the supernatant is colorless. After removing the supernatant, add 0...
Embodiment 3
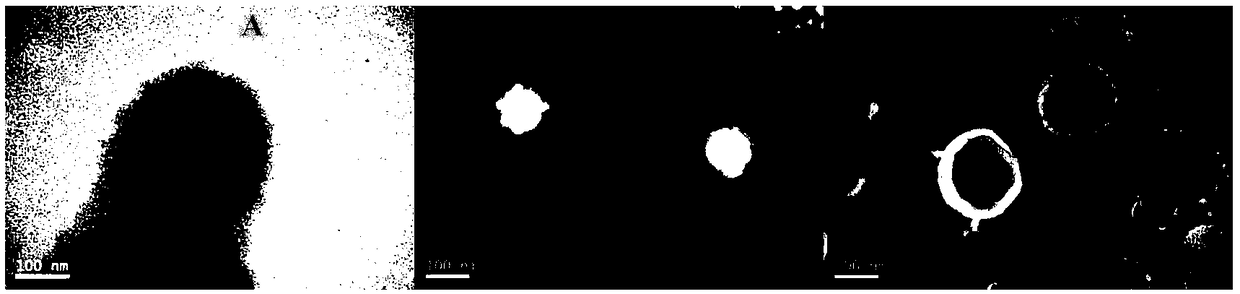
[0058] Example 3. Preparation of erythrocyte membrane vesicles (RVs)
[0059] Take the two tubes of red blood cell membranes prepared according to Example 2, thaw them in a 37°C water bath, first ultrasonicate the red blood cell membranes at a power of 100W and a frequency of 40KHz for 2min, and then use a thin film extruder to pass the vesicles obtained by ultrasound through 800 , 400 and 200nm polycarbonate films were extruded, and the extrusion was repeated 10 times under each particle size to obtain RVs.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com