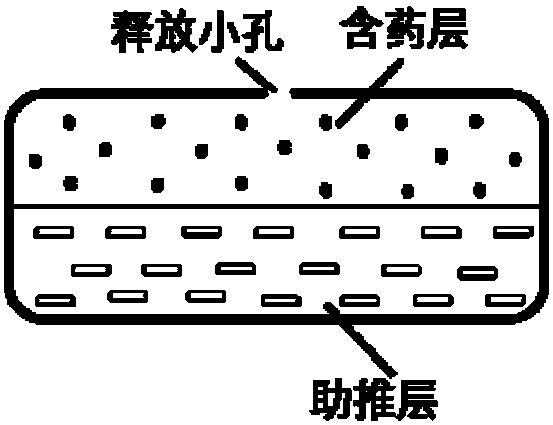
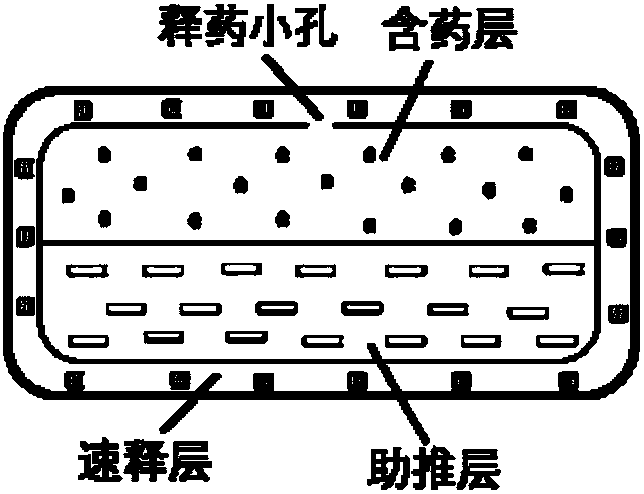
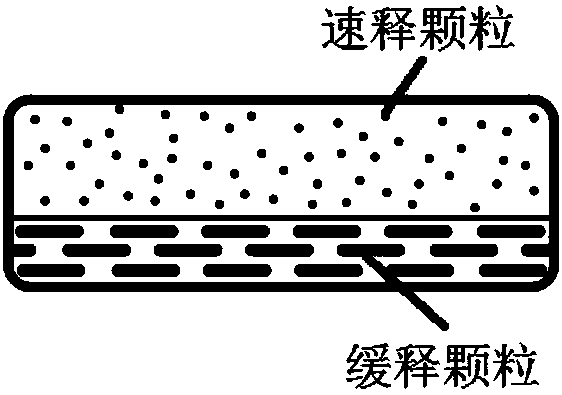
Olaparib oral controlled-release and sustained-release pharmaceutical composition and uses thereof
A controlled-release drug and composition technology, which is applied in drug combination, drug delivery, antineoplastic drugs, etc., can solve the problem of limiting drug efficacy to be fully exerted, there is no research on oral sustained and controlled release preparations of olaparib, steady-state blood drug Problems such as large concentration fluctuation range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Embodiment 1 double-layer osmotic pump controlled-release tablet
[0150]
[0151] Olaparib and copovidone VA64 are prepared as solid dispersion by solvent evaporation method, that is, olaparib and copovidone VA64 are dissolved in ethanol / acetone (25 / 75, v / v) at the same time, and the organic solvent is evaporated under reduced pressure , dried in a vacuum oven, ground and crushed through a 60-mesh sieve, ready for tableting. The obtained solid dispersion can dissolve more than 90% of the active ingredients of the medicine in 30 minutes in water under sink conditions at 37°C and 100 rpm. And under the same conditions, the dissolution of olaparib compound powder 2h is less than 60%.
[0152] Then pass the prescription amount and other auxiliary materials through a 60-mesh sieve and mix them with a three-dimensional mixer at 30 rpm for 25 minutes to obtain a drug-containing layer composition, which is ready for tableting.
[0153] Accurately weigh the auxiliary mater...
Embodiment 1
[0159] Comparative Example 1 Quick Release Capsule Formulation
[0160] Preparation method: Disperse 10% olaparib by weight in Glucire44 / 14 at 65°C, keep stirring for 12 hours, and fill hypromellose capsules (0#) at about 60°C.
[0161]The dissolution rate is determined by the first method device of the dissolution rate determination method (Chinese Pharmacopoeia 2010 edition two appendix X C), under the condition of 37 ℃, with 500mL Tris buffer solution of pH 6.8 as the release medium, the speed is 100 rpm, and the operation is in accordance with the law , After 15, 30, 45, 60, 75, 90, 105, 120min, take 6mL of the solution, centrifuge, take the supernatant as the test solution, and measure the release degree.
[0162] According to the ultraviolet-visible spectrophotometry (Chinese Pharmacopoeia 2010 edition two appendix IVA), measure the absorbance respectively at the wavelength of 278nm, measure the release degree of the capsule.
[0163] see release result Figure 7 . Mo...
experiment Embodiment 1
[0165] The olaparib capsules of Comparative Example 1 and the double-layer osmotic pump controlled-release tablets of Example 1 were administered to full-fed beagle dogs (n=3), respectively, and were delivered with 50 mL of water. For the capsule preparation, 1 mL of blood was collected from the veins of the extremities at 0.25, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 6.0, 8.0, 10, 12 and 24 hours after administration (0h) and after administration, and double-layer osmotic pump controlled release tablets Before administration (0h) and 1.0, 2.0, 4.0, 6.0, 8.0, 10, 12 and 24h after administration, 1mL of blood was collected from the veins of the extremities. The blood samples were centrifuged at 4000rpm for 10min at 4°C, and the upper layer of plasma was collected. For LC-MS blood drug concentration detection, see the results Figure 8 .
[0166] Relative to the T of the capsule formulation 1 / 2 (2.6h), C max (1590.1ng / mL) and AUC 0-h (7155.7h*ng / mL), the T of double-layer osmotic pump...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com