Sustained release formulation of bilobalide-B and preparation process thereof
A ginkgolide, sustained-release preparation technology, applied in the field of pharmaceutical preparations, can solve the problem of no ginkgolide B, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
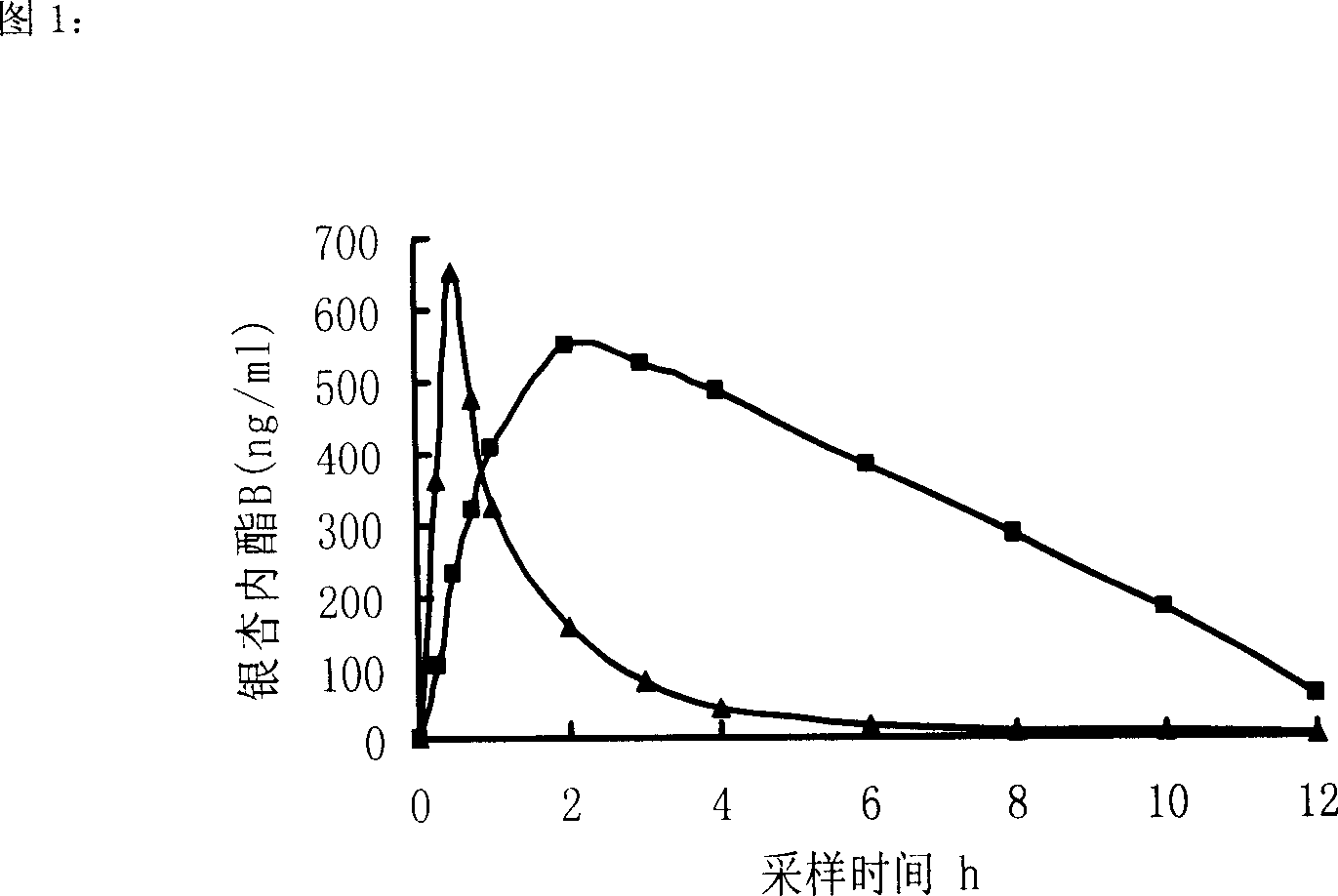
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 ginkgolide B skeleton type sustained-release tablet
[0025] prescription:
[0026] Ginkgolide B 60.0g
[0027] Hydroxypropyl methylcellulose (4000 centipoise) 51.7g
[0028] 3% ethanol solution of hydroxypropyl methylcellulose (50 centipoise) 5.3g (calculated as dry matter)
[0029] Microcrystalline Cellulose 17.5g
[0030] Magnesium stearate 0.9g
[0031] Makes 1000 pieces
[0032] Preparation method: mix ginkgolide B, hydroxypropyl methylcellulose, and microcrystalline cellulose, add 3% hydroxypropyl methylcellulose ethanol solution, mix well, granulate, and dry at 65°C for 12 Hours, add magnesium stearate, mix well, compress into tablets, and obtain.
Embodiment 2
[0033] The preparation of embodiment 2 matrix type sustained-release tablets
[0034] prescription:
[0035] Ginkgolide B 200g
[0036] Microcrystalline Fiber Cord 50.0g
[0037] Carbopol 105.0g
[0038] Polyvinylpyrrolidone K-30 10.5g (calculated as dry matter)
[0039] Magnesium stearate 1.8g
[0040] Makes 1000 pieces
[0041] Preparation method: mix ginkgolide B and polycarboxyethylene, granulate with 5% polyvinylpyrrolidone K-30 aqueous solution, dry at 55°C for 12 hours, add magnesium stearate, mix evenly, and press into tablets. Instantly.
Embodiment 3
[0042] The preparation of embodiment 3 film-controlled sustained-release capsules
[0043] prescription:
[0044] Ginkgolide B 45.0g
[0045] 2% hydroxypropyl methylcellulose (50 centipoise) aqueous solution 6.3 (based on dry matter)
[0046] Trimethylammonium ethyl methacrylate - methacrylate copolymer (Eudragit RS100) 50.0g
[0047] Microcrystalline Cellulose 43.8g
[0048] Polyvinylpyrrolidone K30 ethanol solution 12.5g
[0049]Makes 1000 capsules
[0050] Use methacrylic trimethylammonium ethyl ester-methacrylate copolymer as the coating film material, add a small amount of polyvinylpyrrolidone ethanol solution (PVP-K 30 ) as a porogen, the porogen dissolves in the digestive juice to form micropores on the coating film, and the active ingredient is slowly and steadily released from the micropores.
[0051] Preparation method: Mix the prescription amount of ginkgolide B and microcrystalline cellulose, add hydroxypropyl methylcellulose aqueous solution, mix well, extru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com