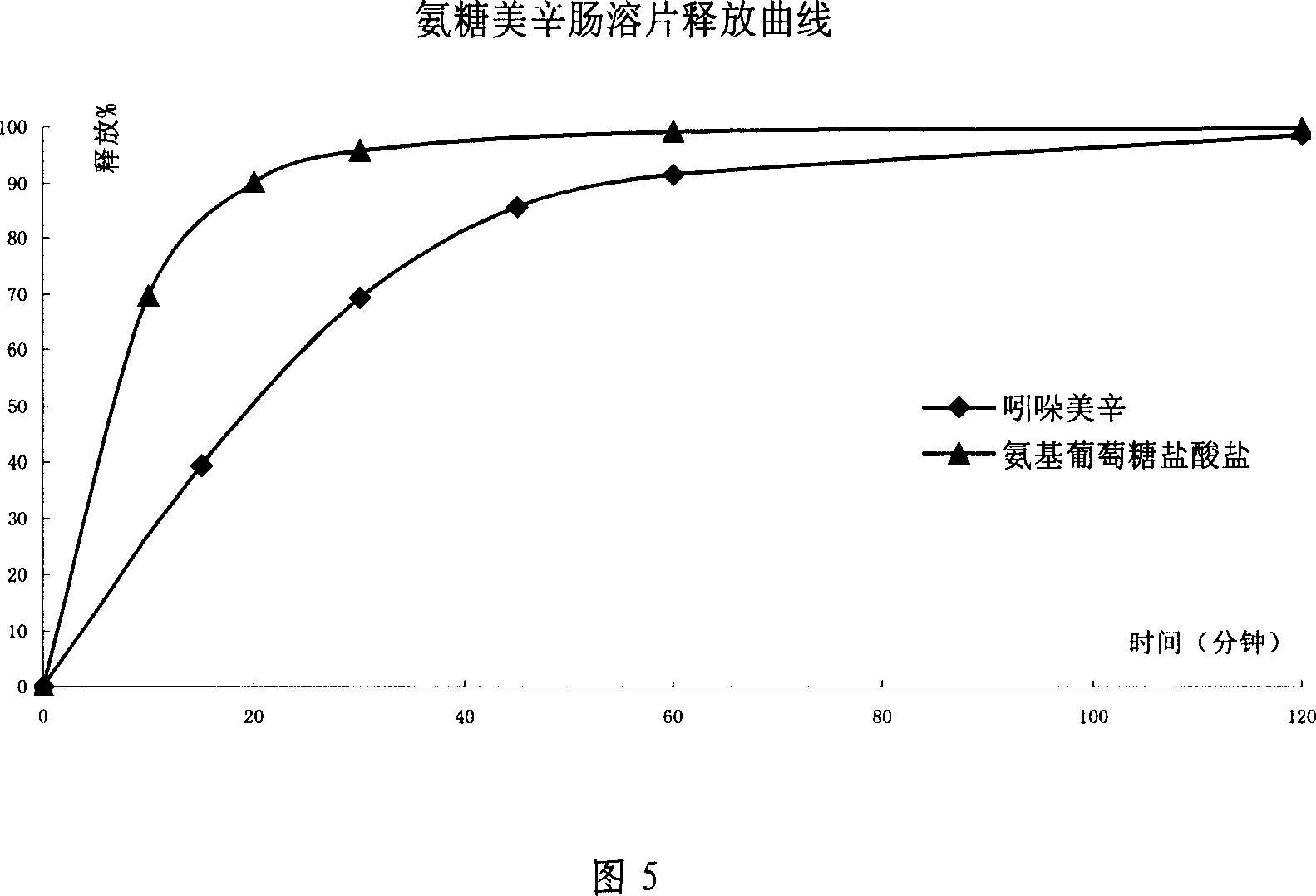
Sustained releasing formulation of compound glucosamine, preparation process and application thereof
A technology of glucosamine salts and sustained-release preparations, applied in anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc. The effect of eliminating toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
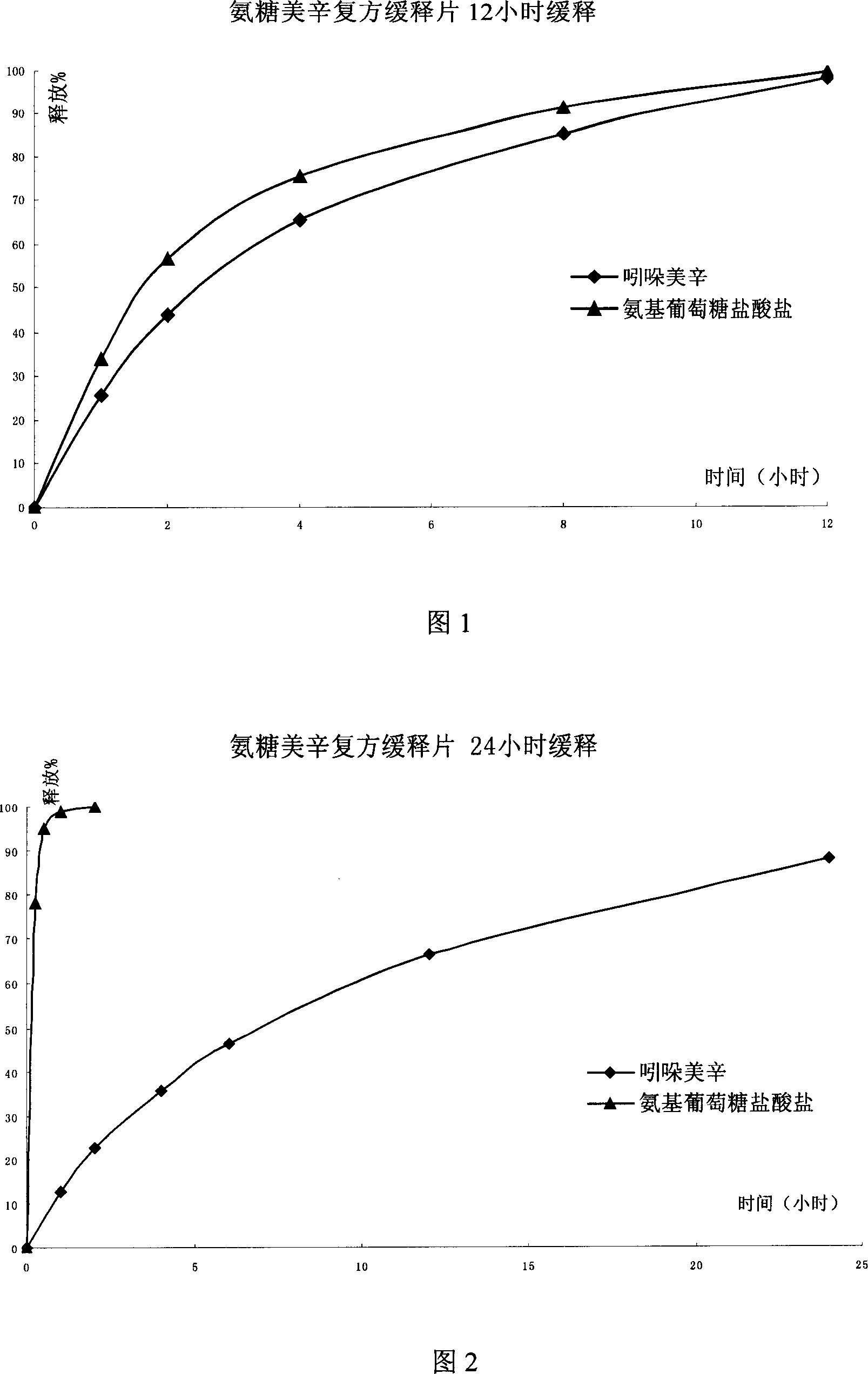
[0045] The preparation of embodiment 1 matrix type sustained-release tablet
[0046] formula:
[0047] Glucosamine Hydrochloride 150.0g
[0048] Indomethacin 50.0g
[0049] Ethylcellulose (100 centipoise) 5.0g
[0050] Hydroxypropyl methylcellulose (4000 centipoise) 5.0g
[0051] Microcrystalline Cellulose 8.0g
[0052] Polyvinylpyrrolidone K30 ethanol solution 3.0g
[0053](K30 is the molecular weight index of the polymer obtained by measuring the viscosity of the polymer)
[0054] Magnesium Stearate 1.0g
[0055] Makes 1000 pieces
[0056] Hydroxypropyl methylcellulose is a hydrophilic skeleton material polymer, and ethyl cellulose is a non-erodible skeleton material. The two are used in combination to control the release of indomethacin and glucosamine hydrochloride in the preparation.
[0057] Preparation:
[0058] 1. Both glucosamine salt and indomethacin are made into sustained-release preparations: the amount of indomethacin, glucosamine hydrochloride, ethyl c...
Embodiment 2
[0062] The preparation of embodiment 2 matrix type sustained-release tablets
[0063] formula:
[0064] Indomethacin 25.0g
[0065] Glucosamine Hydrochloride 75.0g
[0066] Cornstarch 15.0g
[0067] Cellulose acetate phthalate 45.0g
[0068] Microcrystalline Cellulose 15.0g
[0069] 1% aqueous solution of hydroxypropyl methylcellulose 0.5g (calculated as dry matter)
[0070] Magnesium Stearate 1.0g
[0071] Makes 1000 pieces
[0072] Hydroxypropyl methylcellulose is a polymer of hydrophilic skeleton material, which swells with water or digestive juice to form a gel barrier to control the release rate of indomethacin and glucosamine hydrochloride and achieve the purpose of sustained release.
[0073] Preparation:
[0074] 1. Both glucosamine salt and indomethacin are made into sustained-release preparations: the amount of glucosamine hydrochloride and indomethacin, cornstarch, cellulose acetate phthalate required for the above formula of this embodiment , microcrysta...
Embodiment 3
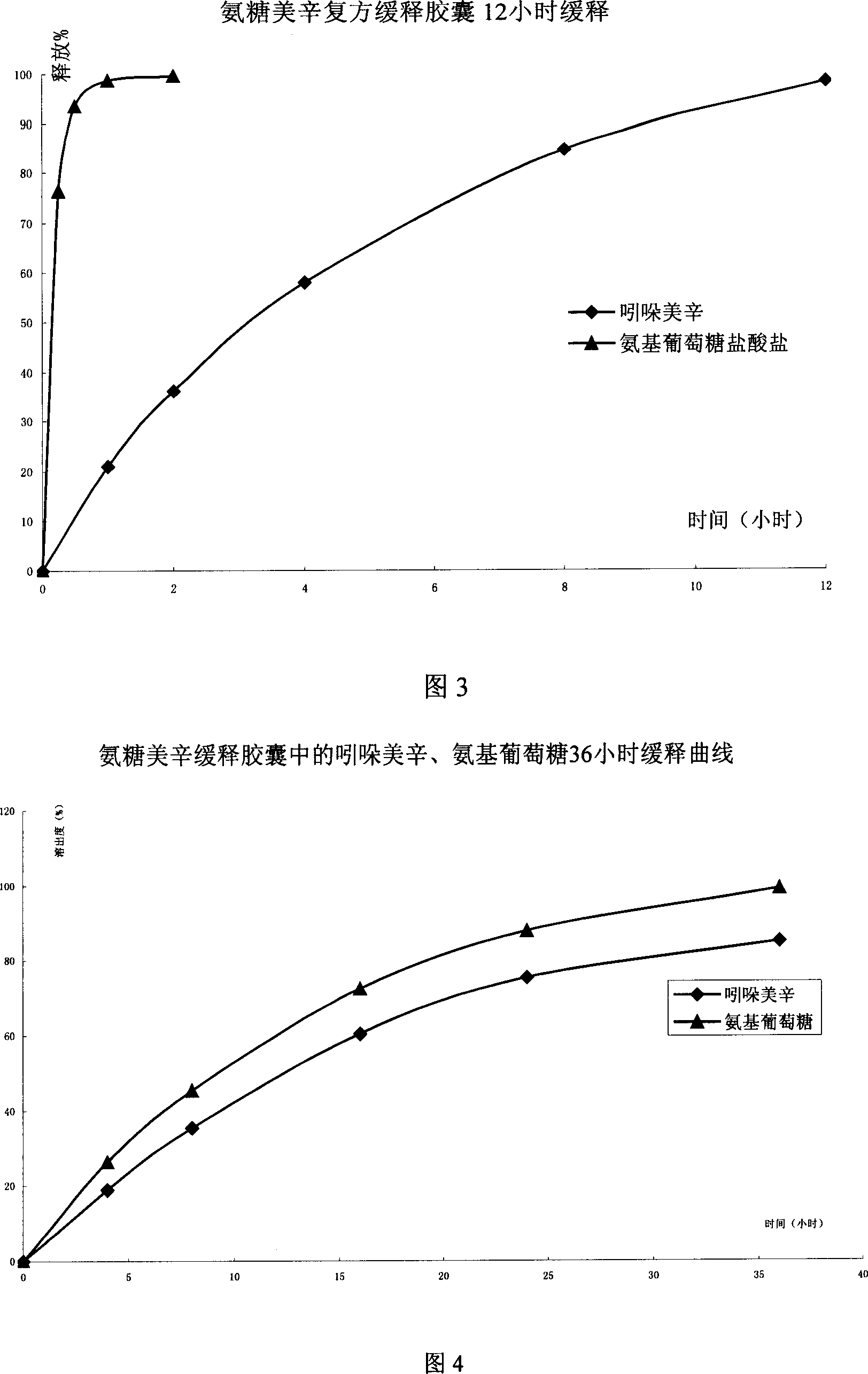
[0077] The preparation of embodiment 3 film-controlled sustained-release capsules
[0078] formula:
[0079] Glucosamine Hydrochloride 150.0g
[0080] Indomethacin 50.0g
[0081] 1.0% hydroxypropyl methylcellulose (4000 centipoise) aqueous solution 2.0g (calculated as dry matter)
[0082] Pregelatinized starch 30.0g
[0083] Trimethylammonium ethyl methacrylate-methacrylate copolymer 21.0g
[0084] (Eudragit RS100)
[0085] Polyvinylpyrrolidone K30 ethanol solution 4.0g
[0086] (K30 is the molecular weight index of the polymer obtained by measuring the viscosity of the polymer)
[0087] Makes 1000 capsules
[0088] Using Eudragit RS100 as the coating material, a small amount of polyvinylpyrrolidone ethanol solution (PVPk30) was added to the coating solution as a porogen. After the porogen dissolved in the digestive juice, micropores were formed on the coating. Slow and smooth release in the hole.
[0089] Preparation:
[0090] 1. Both glucosamine salt and indometh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com