Rosuvastatin calcium sustained-release preparation and preparation method thereof
A technology for rosuvastatin calcium and sustained-release preparations, applied in the field of sustained-release preparations of rosuvastatin calcium and its preparation, capable of solving problems such as rhabdomyolysis, liver toxicity, headache and flu-like symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: Rosuvastatin Calcium Hydrophilic Gel Matrix Sustained Release Tablets
[0014] Prescription composition:
[0015] Rosuvastatin Calcium 5.2mg
[0016] Hypromellose K4M 72mg
[0017] Lactose 82mg
[0018] 10% polyvinylpyrrolidone appropriate amount
[0019] 60% ethanol appropriate amount
[0020] Magnesium Stearate 1.6mg
[0021]
[0022] Tablet weight 160mg
[0023] Preparation process: crush rosuvastatin calcium into a 80-mesh sieve, mix evenly with hypromellose K4M and lactose, moisten with 10% polyvinylpyrrolidone and 60% ethanol, and then granulate, dry at 50°C and then granulate. Add magnesium stearate, mix evenly, and press into tablets.
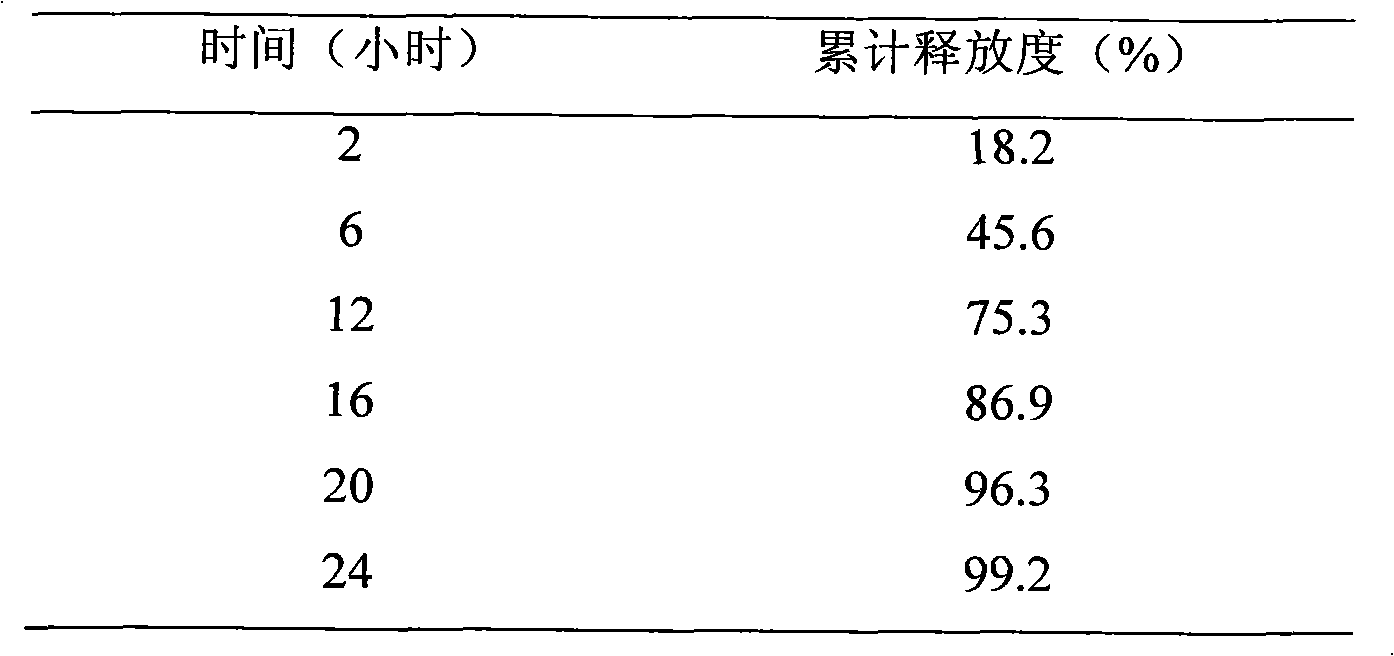
[0024] Release test: In order to investigate the in vitro release characteristics of rosuvastatin calcium sustained-release tablets, according to the Chinese Pharmacopoeia in 2010 two appendix XC, the first method (basket method), with 900ml of water as the dissolution medium, the...
Embodiment 2
[0028] Example 2: Rosuvastatin Calcium Hydrophilic Gel Matrix Sustained Release Tablets
[0029] Prescription composition:
[0030] Rosuvastatin Calcium 5.2mg
[0031] Hypromellose K15M 64mg
[0032] Microcrystalline Cellulose 90mg
[0033] 10% polyvinylpyrrolidone appropriate amount
[0034] 60% ethanol appropriate amount
[0035] Magnesium Stearate 1.6mg
[0036]
[0037] Tablet weight 160mg
[0038] Preparation process: crush rosuvastatin calcium into a 80-mesh sieve, mix evenly with hypromellose K15M and microcrystalline cellulose, moisten with 10% polyvinylpyrrolidone and 60% ethanol, and granulate, dry at 50°C Whole grains, add magnesium stearate, mix evenly, compress into tablets, and obtain.
[0039] Release test: test method is the same as embodiment 1
[0040] The experimental results are shown in Table 2
[0041] Table 2
[0042]
[0043]
Embodiment 3
[0044] Example 3: Rosuvastatin Calcium Hydrophilic Gel Matrix Sustained Release Tablets
[0045] Prescription composition:
[0046] Rosuvastatin Calcium 5.2mg
[0047] Sodium Alginate 58mg
[0048] Microcrystalline Cellulose 96mg
[0049] 10% polyvinylpyrrolidone appropriate amount
[0050] 60% ethanol appropriate amount
[0051] Magnesium Stearate 1.6mg
[0052]
[0053] Tablet weight 160mg
[0054] Preparation process: crush rosuvastatin calcium into a 80-mesh sieve, mix it evenly with sodium alginate and microcrystalline cellulose, moisten it with 10% polyvinylpyrrolidone and 60% ethanol, and then granulate it, dry it at 50°C, and granulate it. Add magnesium stearate, mix evenly, and press into tablets.
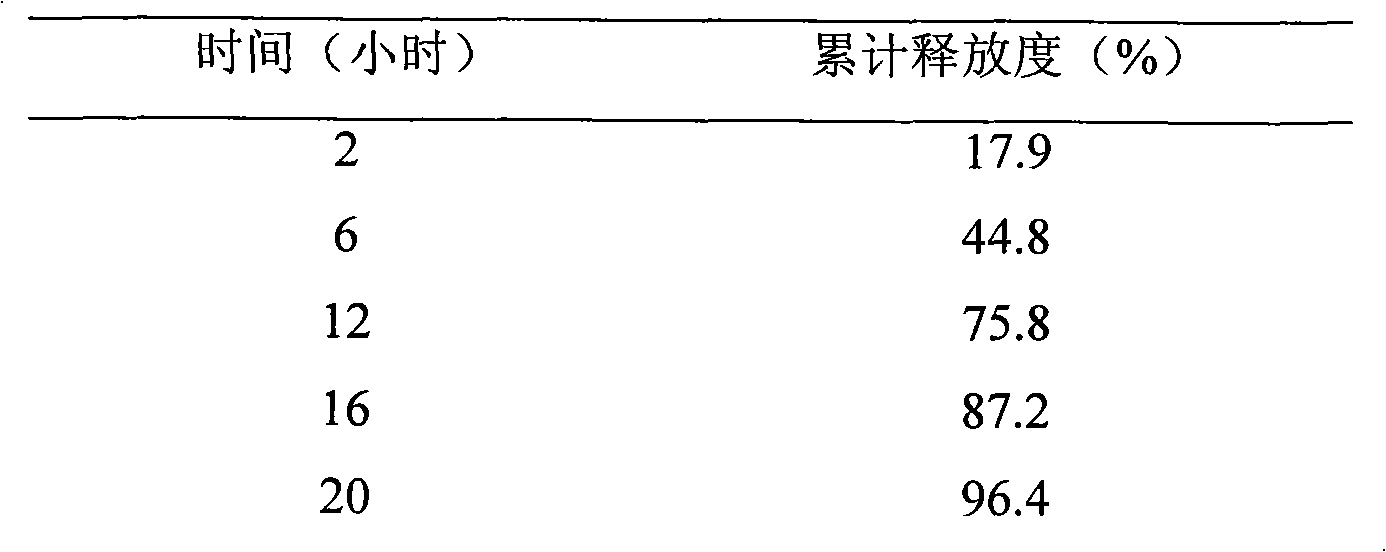
[0055] Release test: test method is the same as embodiment 1
[0056] The experimental results are shown in Table 3
[0057] table 3
[0058]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com