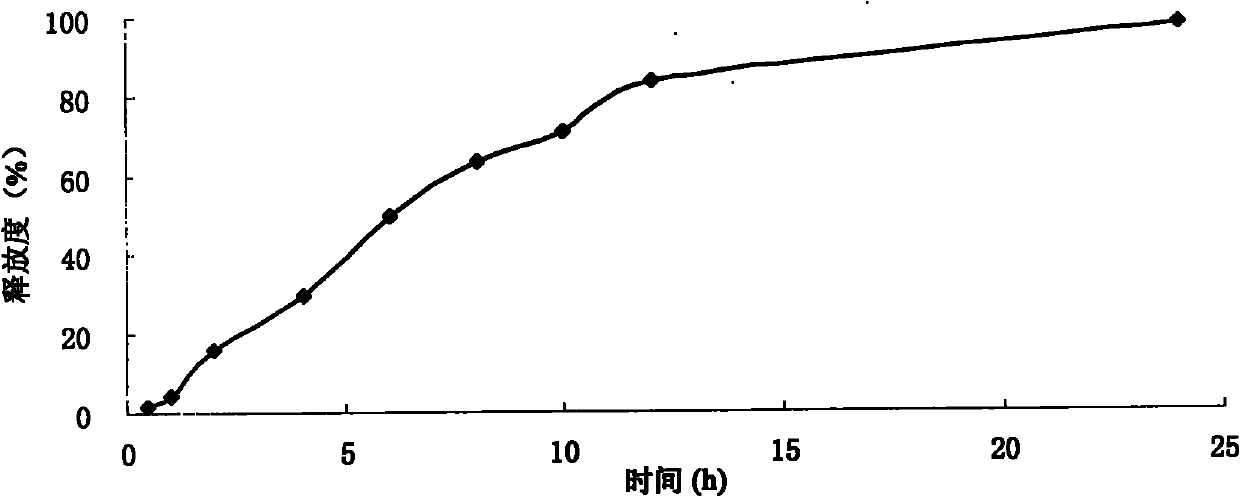
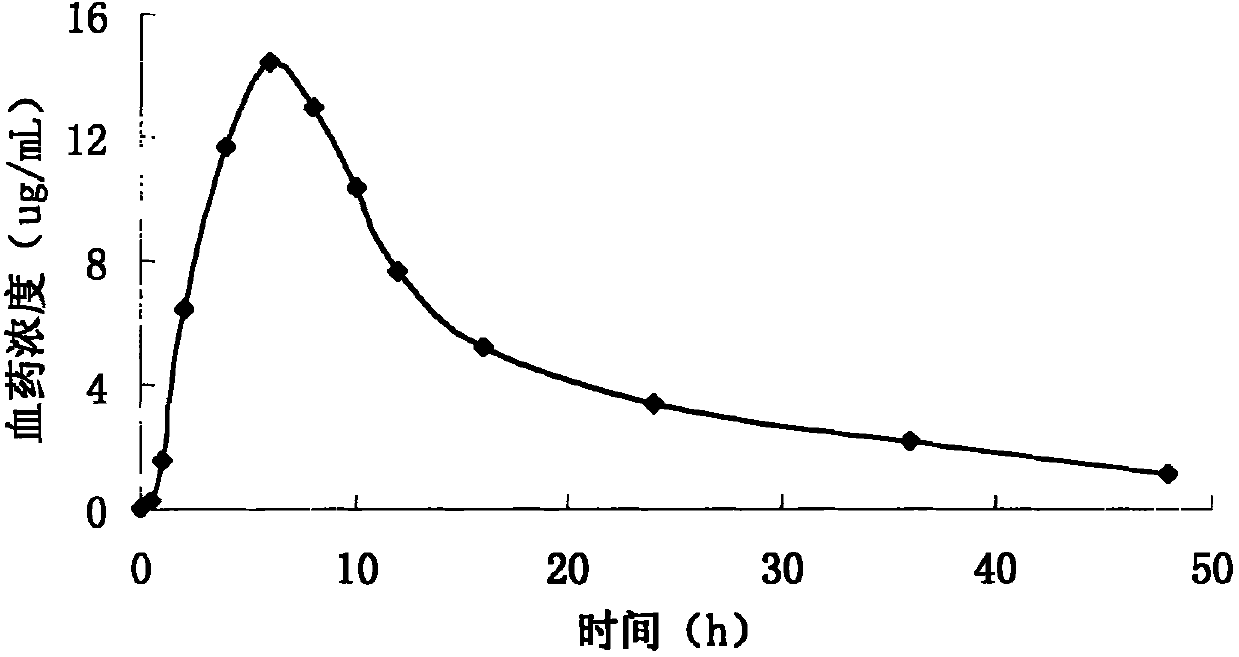
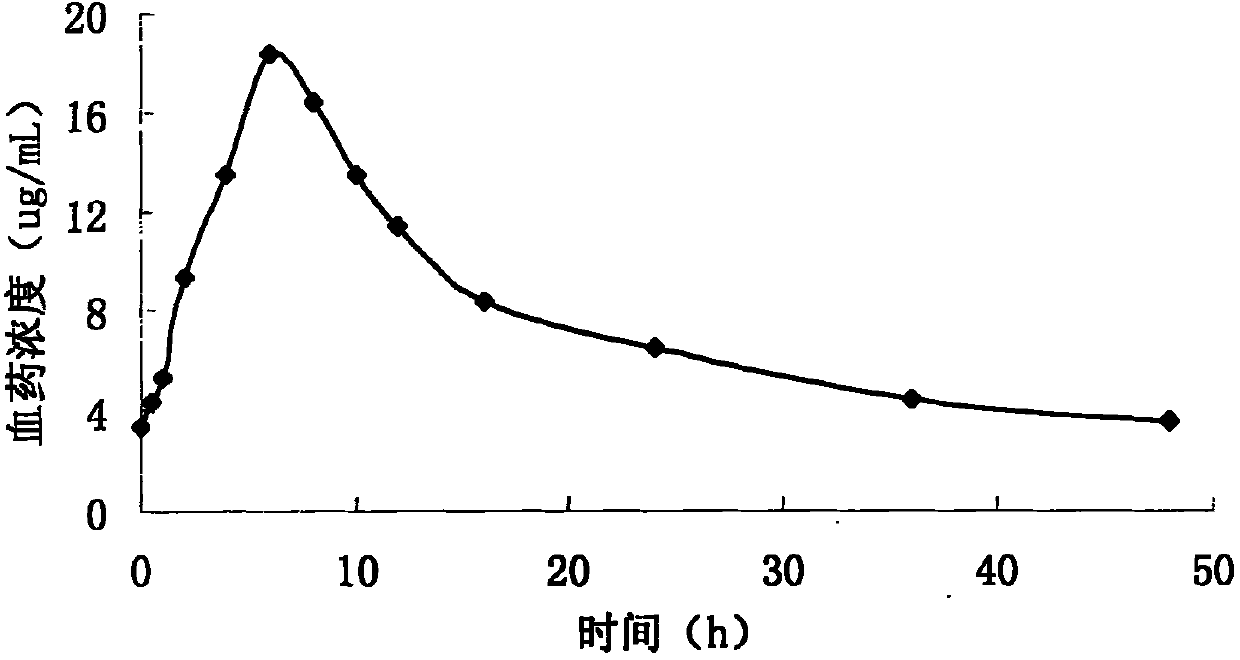
Pyridostigmine bromide sustained-release tablet and preparation method thereof
A clear, tablet-core technology, applied in the field of medicine, can solve problems such as unseen prescriptions and research reports on preparation processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The tablet core is a skeleton tablet, including the following components, and its weight composition or weight percentage is:
[0035] Pyridostigmine bromide 18 parts
[0036] HPMC K15M 12 copies
[0037] Microcrystalline cellulose 3.5 parts
[0038] Lactose 2 parts
[0039] Talcum powder 0.2 parts
[0040] The components contained in the coating solution account for the weight percent of the coating solution as follows:
[0041] Cellulose acetate 3%
[0042] PEG 6000 0.06%
[0043] Talc 0.01%
[0044]The preparation method comprises the following steps: (1) tablet core preparation: weigh pyridostigmine bromide, HPMC, microcrystalline cellulose, lactose and sieve according to tablet core prescription quantity, mix well, use 95% ethanol solution as wetting agent Prepare soft materials, sieve and granulate, dry the wet granules, adjust the granules, add talcum powder and mix evenly, put the above mixture into a tablet machine and compress into tablets to obtain tab...
Embodiment 2
[0046] The tablet core is a skeleton tablet, including the following components, and its weight composition or weight percentage is:
[0047] Pyridostigmine bromide 18 parts
[0048] HPMC K15M 8 parts
[0049] HPMC K15M 8 parts
[0050] 5 parts microcrystalline cellulose
[0051] Lactose 3 parts
[0052] Talcum powder 0.3 parts
[0053] The component that coating liquid contains accounts for the percentage by weight of coating liquid:
[0054] Ethylcellulose 4%
[0055] Polyethylene glycol PEG 0.08%
[0056] Talc 0.02%
[0057] The preparation method is basically the same as in Example 1. Alternatively, the coating is applied with an aqueous dispersion of the coating components.
Embodiment 3
[0059] The tablet core is a skeleton tablet, including the following components, and its weight composition is as follows:
[0060] The tablet core is a skeleton tablet, including the following components, and its weight composition or weight percentage is:
[0061] 9 parts of pyridostigmine bromide
[0062] HPMC K100M 8 parts
[0063] 2 parts microcrystalline cellulose
[0064] 1 part lactose
[0065] Talc powder 0.1 parts
[0066] A coating layer composed of a coating liquid comprising the following components, the weight percentage of the components contained in the coating liquid is:
[0067] Ethylcellulose 2%
[0068] Polyglycol PEG 0.04%
[0069] Talc 0.01%
[0070] The preparation method is basically the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com