Monosialotetrahexosyl ganglioside sodium for injection and preparation method thereof
A technology of ganglioside sodium and monosialic acid, which is applied in the field of medicine, can solve the problems of foreign body sensation, reduce compliance and comfort, and low drug load in patients, so as to reduce foreign body sensation, improve the quality of life, and improve the quality of life. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
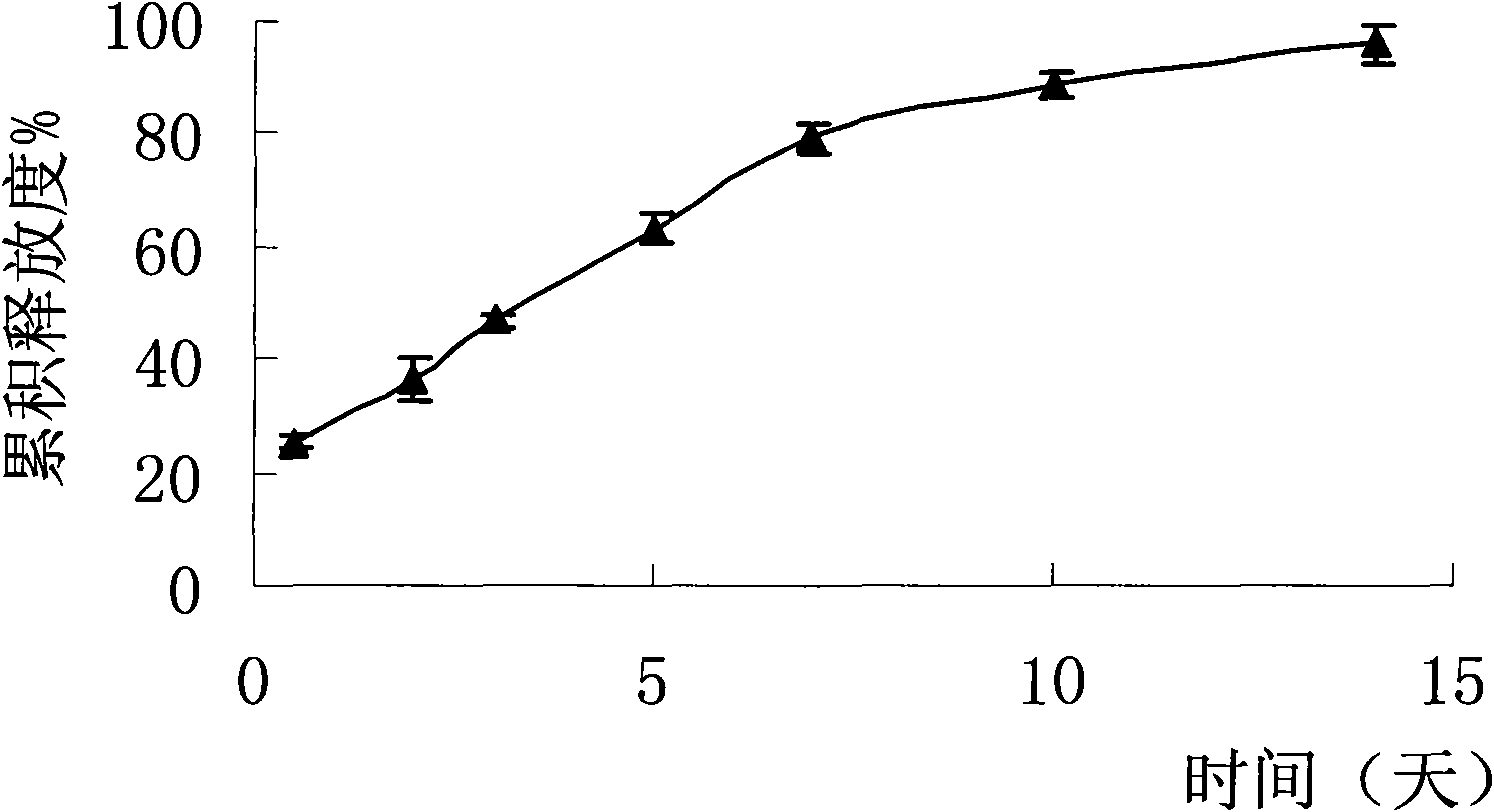
Image
Examples
Embodiment 1
[0019] Embodiment 1, monosialotetrahexosyl ganglioside sodium for injection
[0020] 0.1 parts by weight of monosialotetrahexosyl ganglioside sodium and 0.3 parts by weight of gelatin are dissolved in 4 parts by volume of water, dissolved and mixed as the inner water (W1) phase; 5 parts by weight of polylactic acid- Glycolic acid (the ratio of lactide to glycolide in polylactic acid-glycolic acid is 75 / 25, molecular weight 6000) is dissolved in the dichloromethane of 40 volume parts as oil (O) phase; 5 weight parts of polyethylene Dissolve the alcohol in 500 parts of water for injection at 80°C as the external water (W2) phase; slowly drop the W1 phase into the O phase, emulsify at a high speed of 15000r / min for about 3-5min, cool to 10°C, and use it as the initial phase Milk, and added to the W2 phase, emulsified at 15000r / min high-speed oscillation for about 3-5min, kept at 5000r / min for 4h to evaporate dichloromethane, centrifuged at 10000r / min at high speed, washed 5 times...
Embodiment 2
[0021] Embodiment 2, monosialotetrahexosyl ganglioside sodium for injection
[0022] 0.1 parts by weight of monosialotetrahexosyl ganglioside sodium and 0.3 parts by weight of gelatin are dissolved in 4 parts by volume of water, dissolved and mixed as the inner water (W1) phase; 5 parts by weight of polylactic acid- Glycolic acid (the ratio of lactide and glycolide in polylactic acid-glycolic acid is 75 / 25, molecular weight 20000) is dissolved in the dichloromethane of 40 volume parts as oil (O) phase; 5 weight parts of polyethylene Dissolve the alcohol in 500 parts of water for injection at 80°C as the external water (W2) phase; slowly drop the W1 phase into the O phase, emulsify at a high speed of 15000r / min for about 3-5min, cool to 10°C, and use it as the initial phase Milk, and added to the W2 phase, emulsified at 15000r / min high-speed oscillation for about 3-5min, kept at 5000r / min for 4h to evaporate dichloromethane, centrifuged at 10000r / min at high speed, washed 5 tim...
Embodiment 3
[0023] Embodiment 3, monosialotetrahexosyl ganglioside sodium for injection
[0024]0.1 parts by weight of monosialotetrahexosyl ganglioside sodium and 0.3 parts by weight of gelatin are dissolved in 4 parts by volume of water, dissolved and mixed as the inner water (W1) phase; 5 parts by weight of polylactic acid- Glycolic acid (the ratio of lactide to glycolide in polylactic acid-glycolic acid is 75 / 25, molecular weight 35000) is dissolved in the dichloromethane of 40 volume parts as oil (O) phase; 5 weight parts of polyethylene Dissolve the alcohol in 500 parts of water for injection at 80°C as the external water (W2) phase; slowly drop the W1 phase into the O phase, emulsify at a high speed of 15000r / min for about 3-5min, cool to 10°C, and use it as the initial phase Milk, and added to the W2 phase, emulsified at 15000r / min high-speed oscillation for about 3-5min, kept at 5000r / min for 4h to evaporate dichloromethane, centrifuged at 10000r / min at high speed, washed 5 times...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com