Solid liposome nano granule with antineoplastic activity
A technology of solid lipid nanoparticles and anti-tumor activity, which is applied in the field of solid lipid nanoparticles with anti-tumor activity to achieve the effects of reducing distribution, improving curative effect and increasing uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
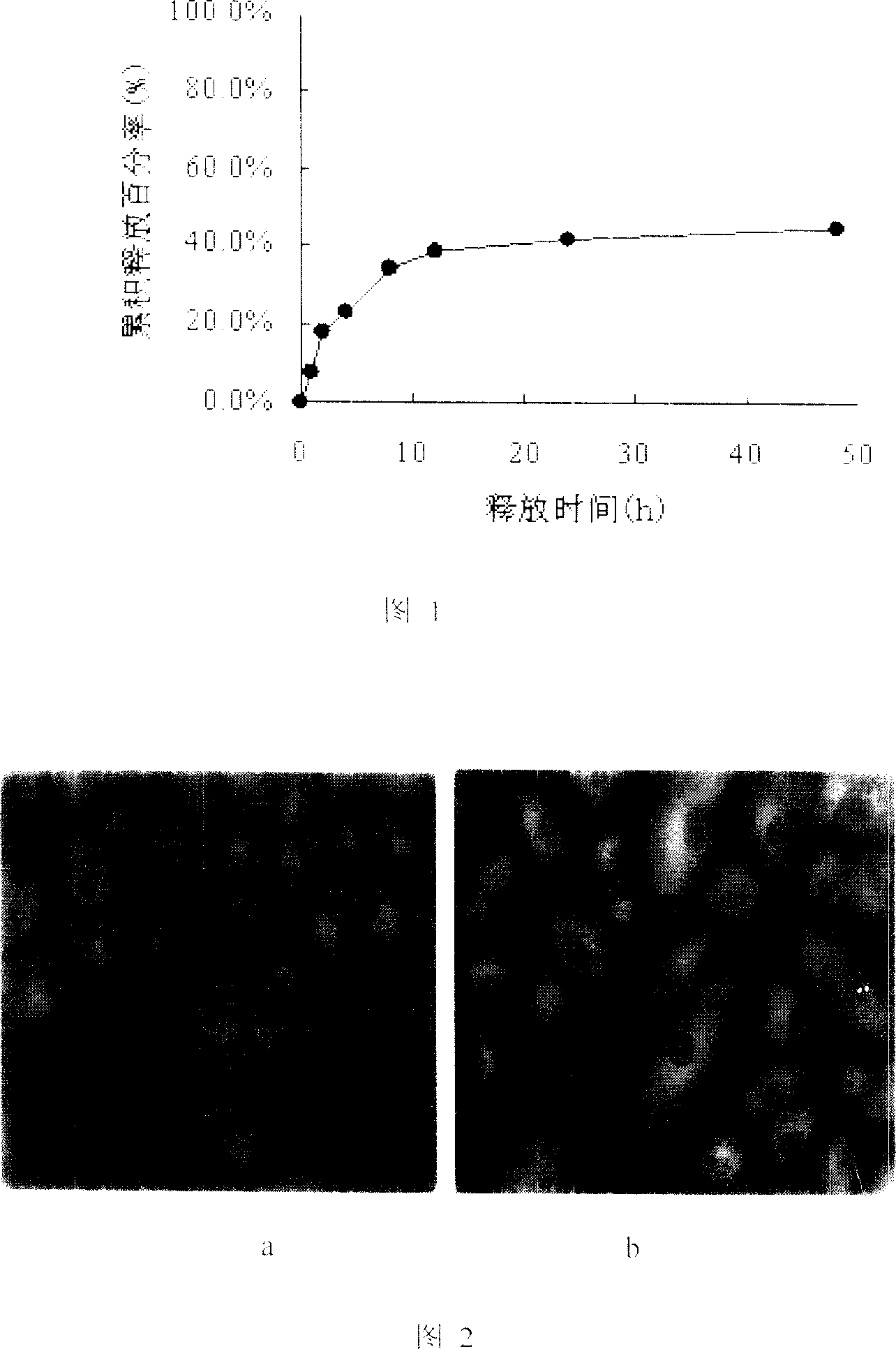
Image
Examples
Embodiment 1
[0012] Example 1: Preparation and application of monoglyceride solid lipid nanoparticles loaded with paclitaxel
[0013] 1) Preparation of paclitaxel monoglyceride solid lipid nanoparticles
[0014] Accurately weigh 30 mg of monoglycerides and 1.5 mg of paclitaxel, place them in 3 mL of absolute ethanol, and dissolve them in a water bath at 70°C. Use distilled water as the dispersed phase and place it in a 70°C water bath. At 400r·min -1 Under mechanical stirring conditions, the organic phase was poured into 30 mL of the dispersed phase and stirred for 5 minutes to obtain a dispersion of solid lipid nanoparticles loaded with paclitaxel monoglyceride. The obtained solid lipid nanoparticle dispersion loaded with paclitaxel monoglyceride used 3mol·L -1 Adjust the pH to 1.2 with HCl solution at 20000r·min -1 Centrifuge for 10 minutes, add 0.1% Poloxamer (w / v) to the precipitate and disperse again, then use 1mol·L -1 Adjust the pH to 7.0 with NaOH solution. The solid lipid nanoparticle...
Embodiment 2
[0026] Example 2: Preparation and application of stearic acid solid lipid nanoparticles loaded with paclitaxel
[0027] 1) Preparation of Paclitaxel Stearic Acid Solid Lipid Nanoparticles
[0028] Accurately weigh 30 mg stearic acid and 1.5 mg paclitaxel, place them in 3 mL absolute ethanol, and dissolve them in a water bath at 70°C. Use distilled water as the dispersed phase and place it in a 70°C water bath. At 400r·min -1 Under mechanical stirring conditions, the organic phase was poured into 30 mL of the dispersed phase and stirred for 5 minutes to obtain a dispersion of solid lipid nanoparticles loaded with paclitaxel stearic acid. The obtained dispersion of solid lipid nanoparticles loaded with paclitaxel stearic acid used 3mol·L -1 Adjust the pH to 1.2 with HCl solution at 20000r·min -1 Centrifuge for 10 minutes, add 0.1% Poloxamer (w / v) to the precipitate and disperse again, then use 1mol·L -1 Adjust the pH to 7.0 with NaOH solution. The solid lipid nanoparticles loaded with...
Embodiment 3
[0033] Example 3: Preparation and application of paclitaxel-loaded glyceryl tristearate solid lipid nanoparticles
[0034] 1) Preparation of Paclitaxel Tristearate Solid Lipid Nanoparticles
[0035] Accurately weigh 30 mg stearic acid and 1.5 mg paclitaxel, place them in 3 mL absolute ethanol, and dissolve them in a water bath at 70°C. Use distilled water as the dispersed phase and place it in a 70°C water bath. At 400r·min -1 Under mechanical stirring conditions, the organic phase was poured into 30 mL of the dispersed phase and stirred for 5 minutes to obtain a dispersion of solid lipid nanoparticles loaded with paclitaxel tristearate. The obtained solid lipid nanoparticle dispersion loaded with paclitaxel tristearate is used 3mol·L -1 Adjust the pH to 1.2 with HCl solution at 20000r·min -1 Centrifuge for 10 minutes, add 0.1% Poloxamer (w / v) to the precipitate and disperse again, then use 1mol·L -1 Adjust the pH to 7.0 with NaOH solution. The solid lipid nanoparticles loaded wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com