Lysozyme cross-linked dextran sustained-release microspheres and preparation method thereof
A technology of cross-linked dextran and slow-release microspheres, which is applied in antibacterial drugs, pharmaceutical formulas, medical preparations of non-active ingredients, etc., to achieve strong biosorption capacity, strong water absorption performance, and significant inhibition effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Preparation of lysozyme cross-linked dextran sustained-release microspheres
[0022] Prescription: Lysozyme 0.05g
[0023] Cross-linked dextran microspheres 1.0g
[0024] Purified water 10ml
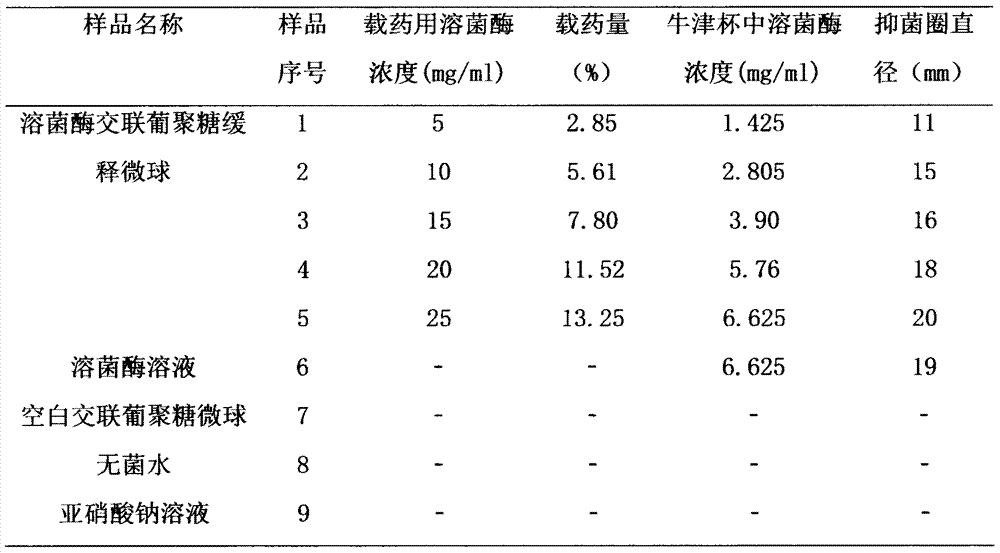
[0025] Preparation method: Dissolve the prescribed amount of lysozyme in purified water in a clean area, add the prescribed amount of cross-linked dextran microspheres, stir at room temperature until the microspheres are completely dispersed, and let stand for 4 hours for adsorption and loading. The microspheres were collected by filtration and vacuum freeze-dried to obtain lysozyme cross-linked dextran sustained-release microspheres with a drug loading of 2.85%.
Embodiment 2
[0026] Example 2 Preparation of lysozyme cross-linked dextran sustained-release microspheres
[0027] Prescription: Lysozyme 0.225g
[0028] Cross-linked dextran microspheres 1.0g
[0029] Purified water 15ml
[0030] Preparation method: Dissolve the prescribed amount of lysozyme in purified water, add the prescribed amount of cross-linked dextran microspheres, stir at room temperature until the microspheres are completely dispersed, and let stand for 12 hours to absorb and load the drug. The microspheres were collected by filtration and vacuum freeze-dried to obtain lysozyme cross-linked dextran sustained-release microspheres with a drug loading of 7.80%.
Embodiment 3
[0031] Example 3 Preparation of lysozyme cross-linked dextran sustained-release microspheres
[0032] Prescription: Lysozyme 0.5g
[0033] Cross-linked dextran microspheres 2.0g
[0034] Purified water 20ml
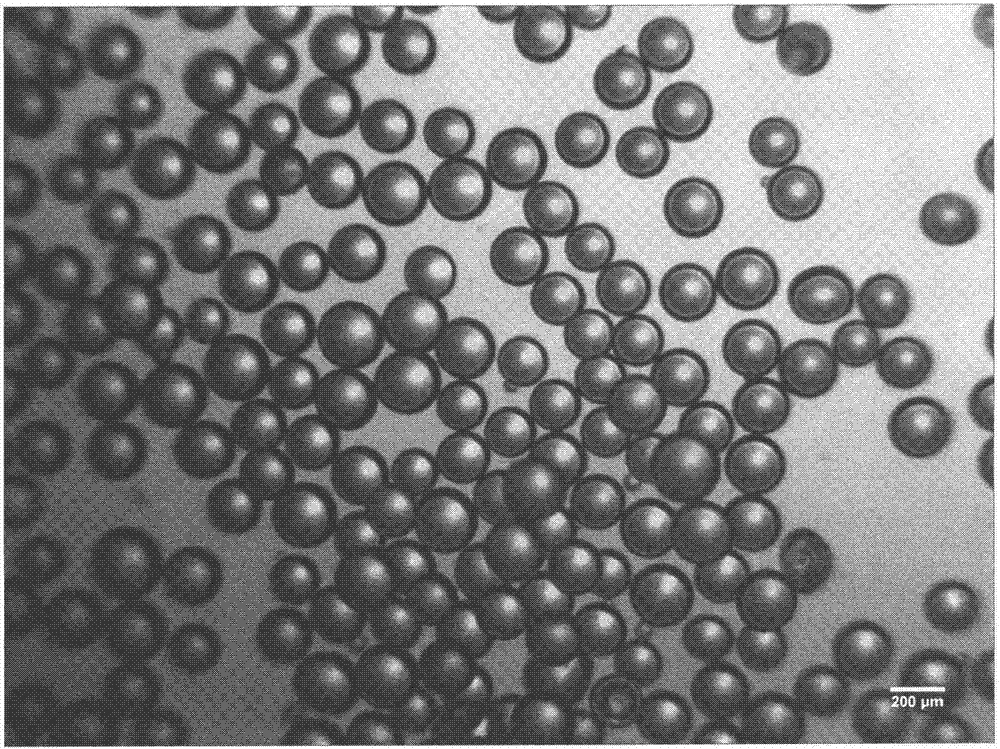
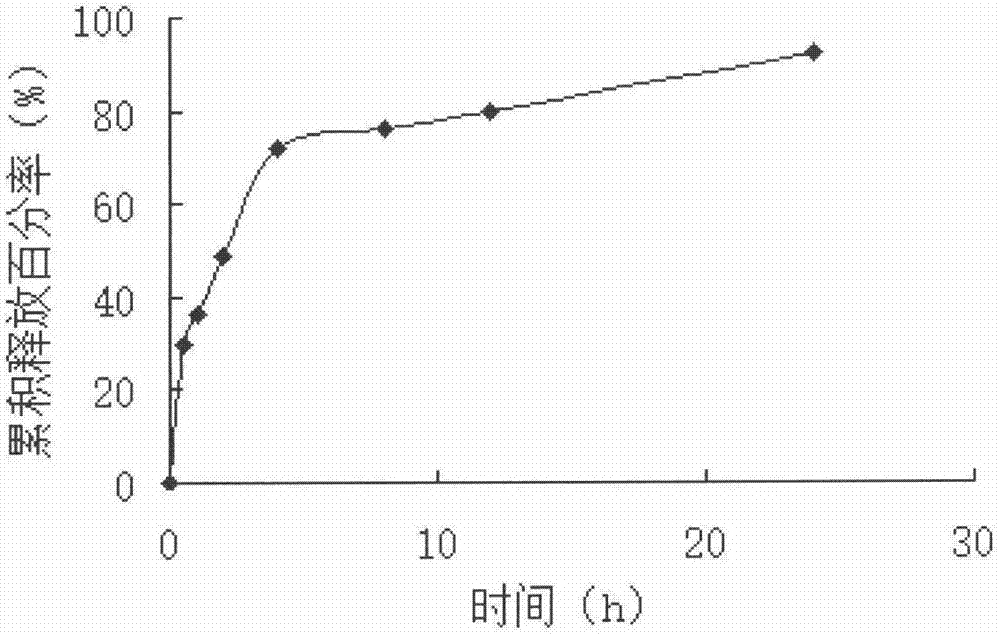
[0035] Preparation method: Dissolve the prescribed amount of lysozyme in purified water, add the prescribed amount of cross-linked dextran microspheres, stir at room temperature until the microspheres are completely dispersed, and let stand for 24 hours to absorb and load the drug. Microspheres were collected by filtration, and vacuum freeze-dried to obtain lysozyme cross-linked dextran sustained-release microspheres (see figure 1 ), the drug loading is 13.25%, the average particle diameter is 207um, and the swelling degree is 8.9ml / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com