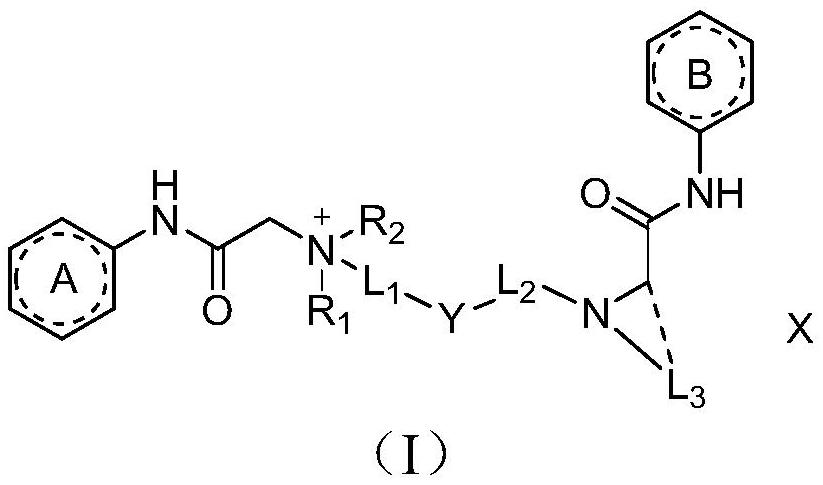
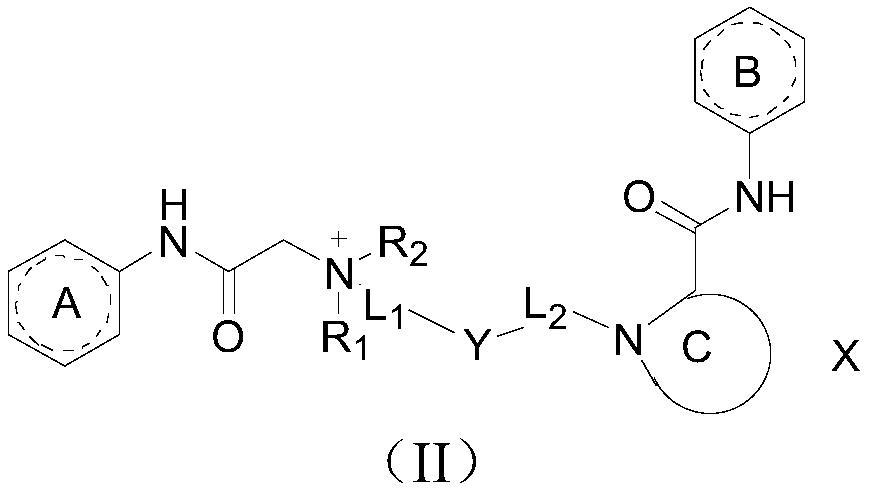
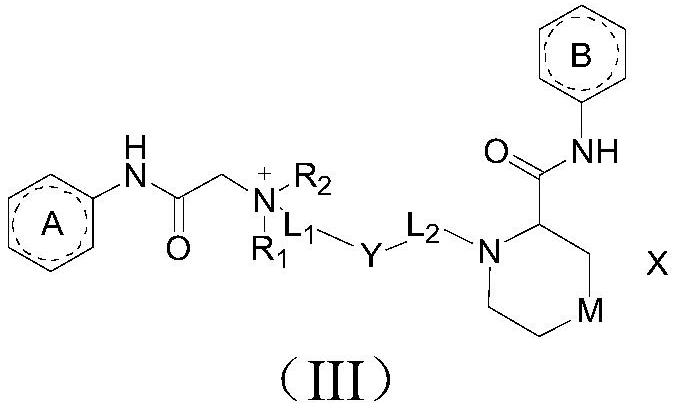
Amide compound, preparation method and application thereof
A compound and solvate technology, applied in the field of drug invention, can solve the problems of inability to completely withdraw opioid analgesics, weak and unsatisfactory sustained analgesic effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Embodiment 1, the preparation of compound 1
[0093]Mix 234mg of lidocaine free base (CAS: 137-58-6) with 5mL of bromoethanol, and stir overnight in a closed reactor at 80°C. The next day, drop the reaction solution into 30mL of diethyl ether, precipitate a solid, and filter to obtain crude product A.
[0094] Mix 232mg of 2-piperidinecarboxylic acid 2,6-dimethylphenylamide (CAS: 15883-20-2) and 181mg of 5-bromo-pentanoic acid in 10mL of acetonitrile, add 400mg of anhydrous potassium carbonate, and stir at 50°C After 6 hours, filter, adjust the pH to 2-3 with 10% methanolic hydrochloric acid solution, evaporate the filtrate to dryness, and obtain crude product B.
[0095] Mix crude product A and crude product B in 10 mL of DMF, drop into 5 mL of DMF solution containing 270 mg of dicyclohexylcarbodiimide (DCC), react at room temperature for 2 hours, evaporate the solvent under reduced pressure, add 20 mL of 1N hydrochloric acid to the residue, stir and Use activated car...
Embodiment 2
[0098] Embodiment 2, the preparation of compound 6
[0099] Dissolve 234 mg of lidocaine free base (CAS: 137-58-6) and 181 mg of methyl 4-bromobutyrate (CAS: 4897-84-1) in 10 mL of acetonitrile, stir at 60°C overnight, and evaporate the solvent to dryness the next day. Add 20 mL of water and 0.2 g of sodium hydroxide, stir at room temperature for 3 hours, adjust the pH value to 2-3 with 1N hydrochloric acid, and evaporate the solvent to dryness under reduced pressure to obtain crude product A.
[0100] 232 mg of 2-piperidinecarboxylic acid 2,6-dimethylphenylamide (CAS: 15883-20-2) was mixed with 139 mg of 3-bromo-propanol (CAS: 627-18-9) in 10 mL of acetonitrile, 70 Stir overnight at ℃, and evaporate acetonitrile to dryness under reduced pressure to obtain crude product B.
[0101] Mix crude product A and crude product B in 10 mL of DMF, drop into 5 mL of DMF solution containing 270 mg of dicyclohexylcarbodiimide (DCC), react at room temperature for 2 hours, evaporate the sol...
Embodiment 3
[0104] Embodiment 3, the preparation of compound 8
[0105] Dissolve 234mg of lidocaine free base (CAS: 137-58-6) and 153mg of methyl bromoacetate (CAS: 96-32-2) in 10mL of acetonitrile, stir overnight at 60°C, evaporate the solvent to dryness the next day, add 20mL of water and 0.2g sodium hydroxide, stirred at room temperature for 3 hours, adjusted the pH value to 2-3 with 1N hydrochloric acid, and evaporated the solvent under reduced pressure to obtain crude product A.
[0106] Mix 232mg of 2-piperidinecarboxylic acid 2,6-dimethylphenylamide (CAS: 15883-20-2) and 125mg of bromoethanol in 10mL of acetonitrile, stir overnight at 50°C, and evaporate the acetonitrile to dryness under reduced pressure to obtain crude product B .
[0107] Mix crude product A and crude product B in 10 mL of DMF, drop into 5 mL of DMF solution containing 270 mg of dicyclohexylcarbodiimide (DCC), react at room temperature for 2 hours, evaporate the solvent under reduced pressure, add 20 mL of 1N hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com