Bis-[6-oxa-(2-carboxylbenzenesulfonyl-butanedioic acid 1,4 monoester-4)-beta-cyclodextrin, preparation method and application thereof
A technology of carboxybenzenesulfonyl chloride and benzenesulfonyl, applied in bis-[6-oxo-(2-m-carboxybenzenesulfonyl-succinic acid 1,4 monoester-4)]-β-cyclodextrin and In the field of preparation and application, it can solve the problems of low production cost, insufficient separation, low solubility of β-CD and application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Embodiment one β-CD-B 2 Preparation and application effect of chiral HPCE column
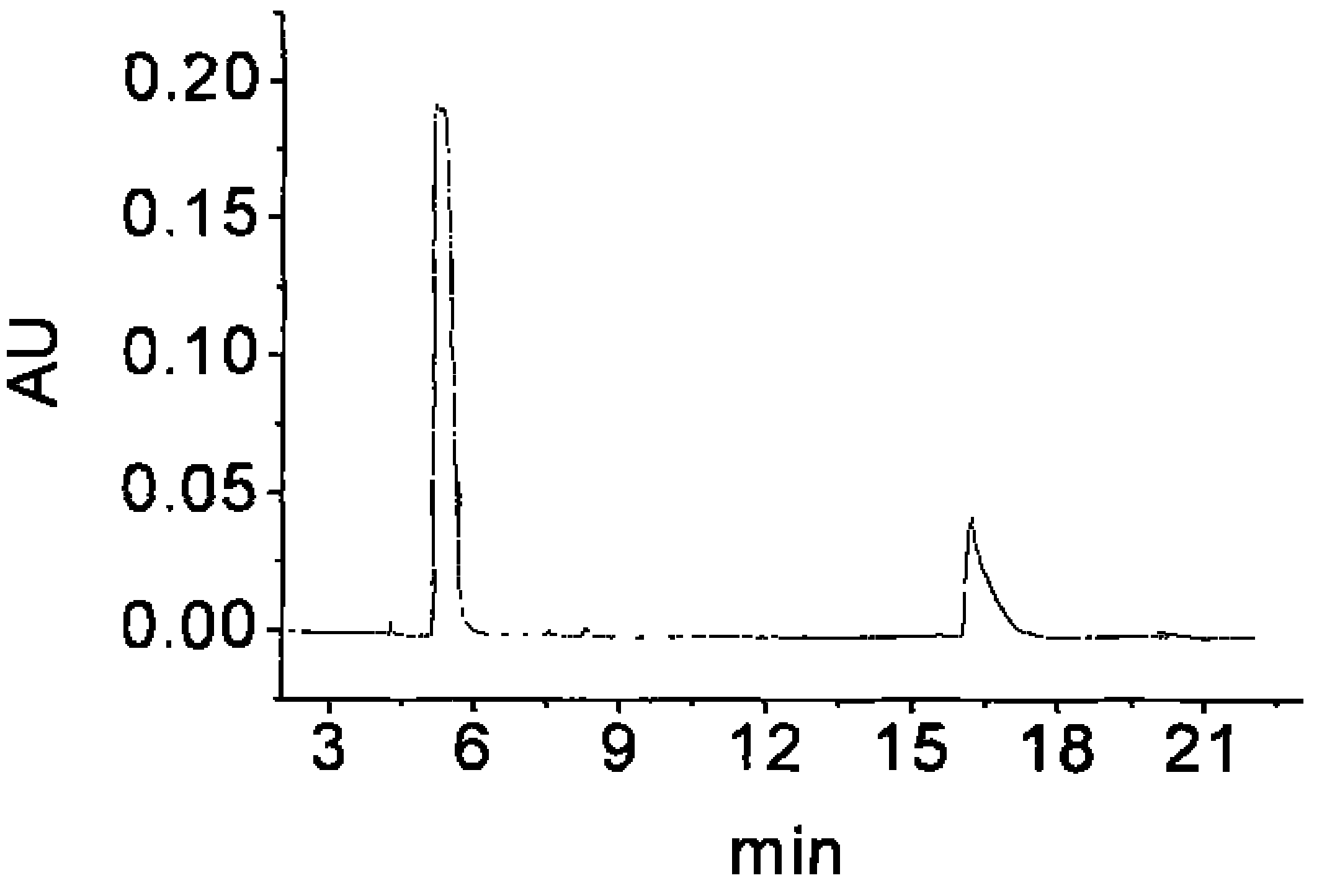
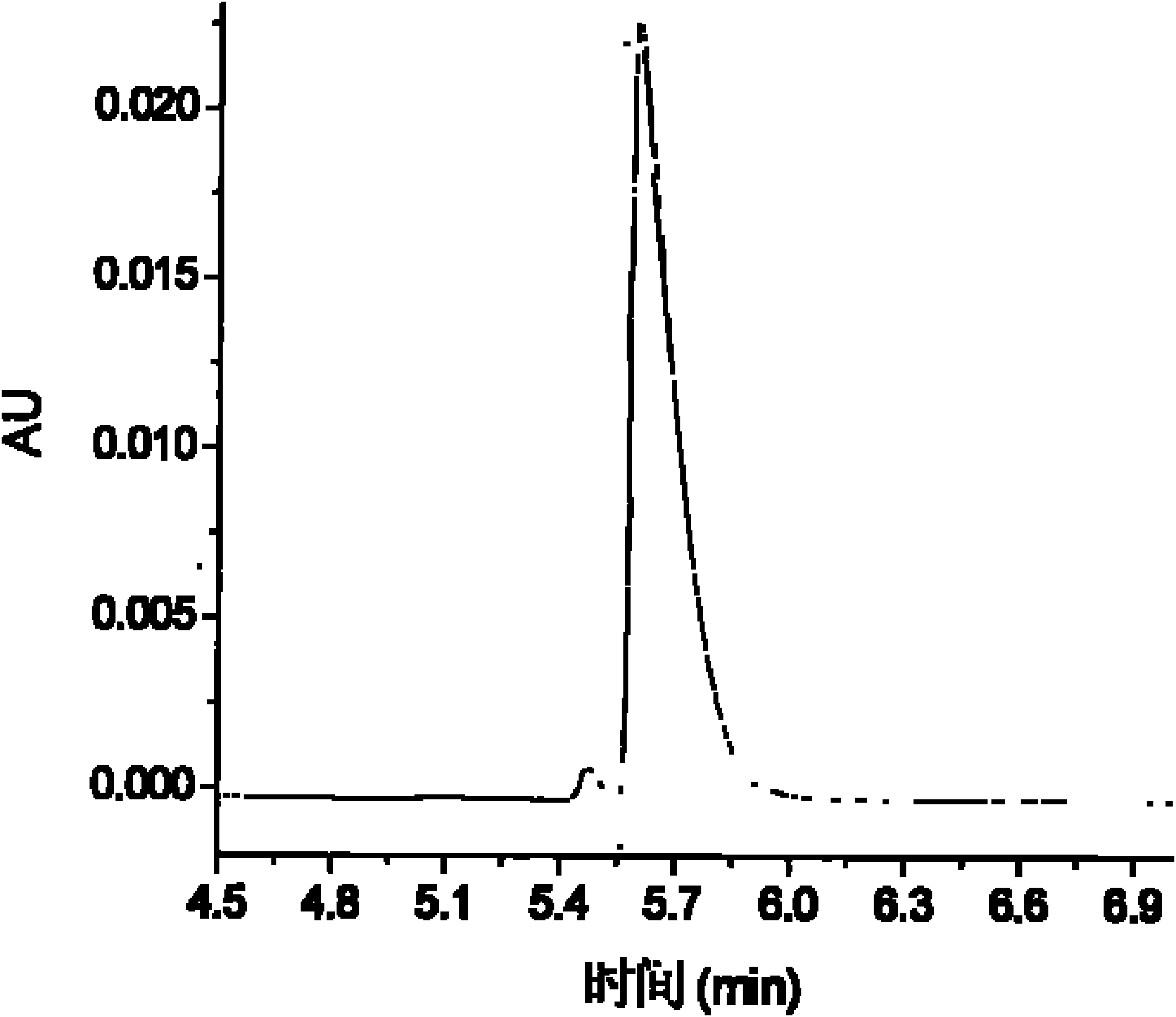
[0140] Using a new chiral separation material synthesized by ourselves - β-CD-B 2 Prepare high-efficiency capillary electrophoresis HPCE column, optimize the preparation conditions, and use scanning electron microscopy to confirm that the inner surface of the capillary column has been bonded to β-CD-B 2 . Specific method: A 75 μm chiral capillary electrophoresis column was prepared by combining physical coating, chemical bonding and monolithic column preparation. Use a static coating device to pretreat the capillary electrophoresis column by chemical bonding to expose more silicon hydroxyl groups on the inner wall of the tube. According to the principle of sol-gel, choose a suitable "arm" - coupling agent, Connect the inner wall of the column with the chiral selector——β-CD derivative, use β-CD-B 2 Bonded capillary electrophoresis columns. The method for preparing the chiral capillary...
Embodiment 2
[0159] Embodiment two uses chiral β-CD-B 2= Column Separation of Caine-like Chiral Drugs
[0160] The chiral drugs procaine hydrochloride and bupivacaine hydrochloride are common local anesthetics and antiarrhythmic drugs. Samir Cherkaoui et al. used different β-CD derivatives to coat HPCE columns and separated bupivacaine hydrochloride by HPCE-mass spectrometry (MS). Caine, lidocaine, mebivacaine, ketamine, the chiral separation of the four drugs can be achieved within 12 minutes. Ivanildo Joséda Silva et al. used HPLC to separate bupivacaine with self-made compound as stationary phase and n-hexane / 2-propanol / acetonitrile / triethylamine as mobile phase. The separation of the two enantiomers was achieved within 15 minutes. The degree is 3.50.
[0161] This embodiment uses the prepared β-CD-B 2 Bonded HPCE chiral column to separate procaine hydrochloride and bupivacaine hydrochloride two chiral drugs of caine. At 8%, 12% and 20% β-CD-B 2 The baseline separation of bupivacai...
Embodiment 3
[0190] Example 3 β-CD-B 2 Separation of chiral drugs in HPCE as a chiral stationary phase
[0191] 1 Main instruments and reagents
[0192] 1.1 The instrument is the same as before. 1.2 Reagents
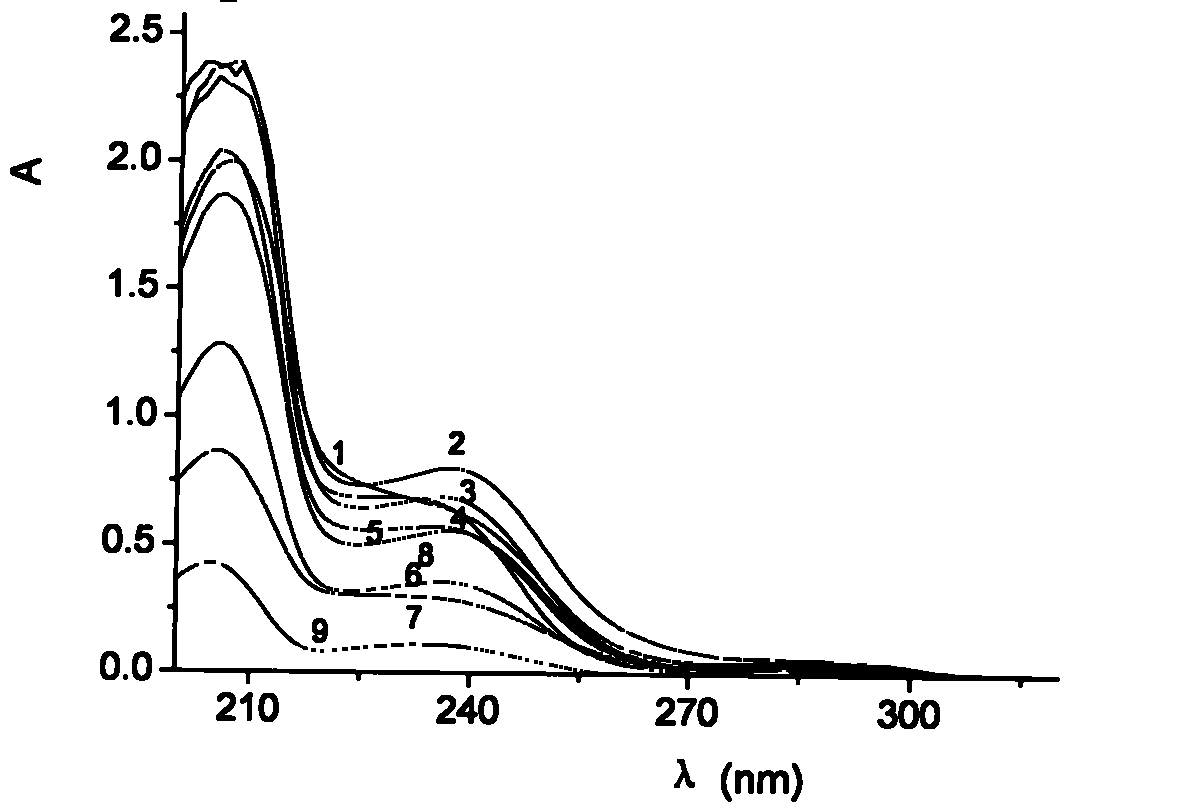
[0193] Chlorpheniramine injection Chlorpheniramine (drugs, Hubei Huazhong Pharmaceutical Co., Ltd., chlorpheniramine maleate, reference substance, 100mg, China Institute for the Control of Pharmaceutical and Biological Products), anisodamine (drugs, Hangzhou Minsheng Pharmaceutical Group Co., Ltd. company); propafenone (drug, Jiangsu Yunyang Group Pharmaceutical Co., Ltd.); DL-phenylglycine (purity: 99%) (Shanghai Qiude Biochemical Co., Ltd.); D.L-phenylglycine (purity: 98.5%); Tris (Tris) AR, Amreso packing; H 3 PO 4 AR (Tianjin Jinnan Chemical Reagent Factory); Citric Acid AR (Shanghai Chemical Reagent Company, Sinopharm Group); Boric Acid AR (China Yunling Chemical Factory); NaOHAR (Tianjin Kemiou Chemical Reagent Co., Ltd.) Chiral Drug Structural Formula as follows:
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com