Skin topical preparation containing bupivacaine or pharmaceutical salt thereof
A technology of bupivacaine and external preparations, which is applied in the field of pharmaceutical preparations, and can solve the problems of reducing the peeling strength of patches, inconvenient industrial production, and reduced stability of patches.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
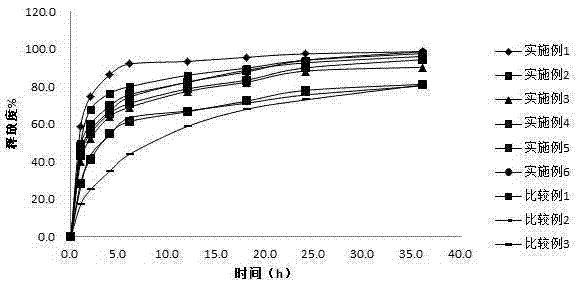
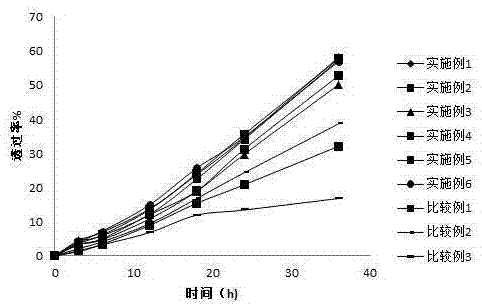
Examples
Embodiment 1
[0024]
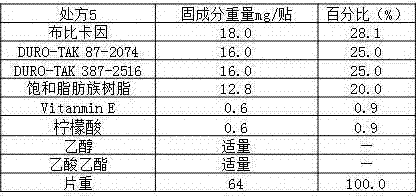
[0025] Preparation method: add the prescribed amount of bupivacaine into an appropriate amount of ethyl acetate, and mix to obtain the main drug mixture; mix the prescribed amount of colloid evenly, add the main drug solution into the colloid, stir, and mix evenly; The prepared paste is evenly spread on the anti-adhesive layer, covered with a backing layer, and cut to the required size after the ethyl acetate volatilizes.
Embodiment 2
[0027]
[0028] Note: The volume ratio of ethyl acetate and ethanol is 1:1 to prepare a mixed solution.
[0029] Preparation method: add the prescribed amount of terpene resin into a certain amount of mixed solution of ethyl acetate and ethanol, and stir evenly; add the prescribed amount of bupivacaine into a certain amount of mixed solution of ethyl acetate and ethanol, and stir evenly , to obtain the main drug solution; add the mixed solution obtained above into a container with colloid, continue to stir, and after stirring evenly, apply the prepared paste evenly on the anti-adhesive layer, cover the backing layer, and wait for ethyl acetate After the ester and ethanol volatilize, it is cut to the required size.
Embodiment 3
[0031]
[0032] Note: The volume ratio of ethyl acetate and ethanol is 1:1 to prepare a mixed solution.
[0033] Preparation method: add the prescribed amount of terpene resin into a certain amount of mixed solution of ethyl acetate and ethanol, and stir evenly; add the prescribed amount of bupivacaine into a certain amount of mixed solution of ethyl acetate and ethanol, and stir evenly , to obtain the main drug solution; add the mixed solution obtained above into a container with colloid, continue to stir, and after stirring evenly, apply the prepared paste evenly on the anti-adhesive layer, cover the backing layer, and wait for ethyl acetate After the ester and ethanol volatilize, it is cut to the required size.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com