A kind of preparation method of bupivacaine multivesicular liposome and bupivacaine multivesicular liposome preparation
A multivesicular liposome and bupivacaine technology, which is applied in the direction of liposome delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of sudden release and affect the therapeutic effect of drugs , the drug can not achieve the expected long-acting sustained-release effect and other problems, to achieve the effect of round shape and improve the long-acting sustained-release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The preparation method of the bupivacaine multivesicular liposome provided by the present embodiment comprises the following steps:
[0070] 1 Formation of water-in-oil colostrum (W / O)
[0071] Mix 5mL of the first water phase and 25mL of the oil phase (the volume of the first water phase and the oil phase is 1:5), place in an ice bath, and shear at a speed of 16000rpm for 9min to form water-in-oil colostrum.
[0072] Wherein, the composition of the first aqueous phase is as follows:
[0073] The solvent is water;
[0074] The solutes were: 60 mg / mL bupivacaine, 150 mM glucuronic acid, 15 mM hydrochloric acid, and 20 mM phosphoric acid.
[0075] The composition of the oil phase is as follows:
[0076] Organic solvent is dichloromethane;
[0077] The solutes were: 18.6 mM DEPC, 4.2 mM DPPG and 30 mM cholesterol.
[0078] 2 plus neutral fat (TC)
[0079] Add 9 mg of TC to the colostrum obtained from the above steps (the mass ratio of the amount of TC added to the bu...
Embodiment 2
[0102] Such as Figure 5 As shown, the present embodiment provides the bupivacaine multivesicular liposome preparation device suitable for the preparation method of the bupivacaine multivesicular liposome described in Example 1, which includes:
[0103] The first water phase storage tank 1, the oil phase storage tank 2, the neutral fat storage tank 3, the first water phase storage tank control valve 4, the oil phase storage tank control valve 5, the neutral fat storage tank control valve 6, temperature control Device 7, colostrum reaction tank 8, colostrum flow rate control valve 9, second water phase storage tank 10, second water phase storage tank control valve 11, double milk reaction tank 12, double milk flow rate control valve 13, three-way device 14, a nitrogen generator 15, a nitrogen control valve 16, a third water phase storage tank 17, a third water phase storage tank control valve 18 and a multivesicular liposome receiving tank 19.
[0104] Wherein, the colostrum r...
Embodiment 3
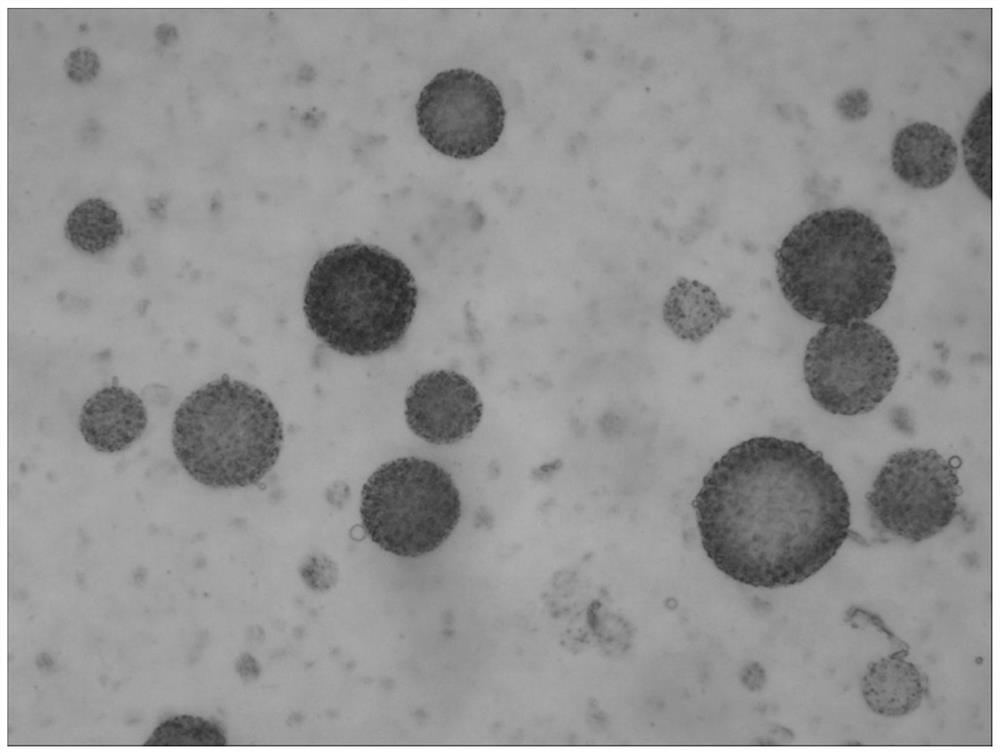
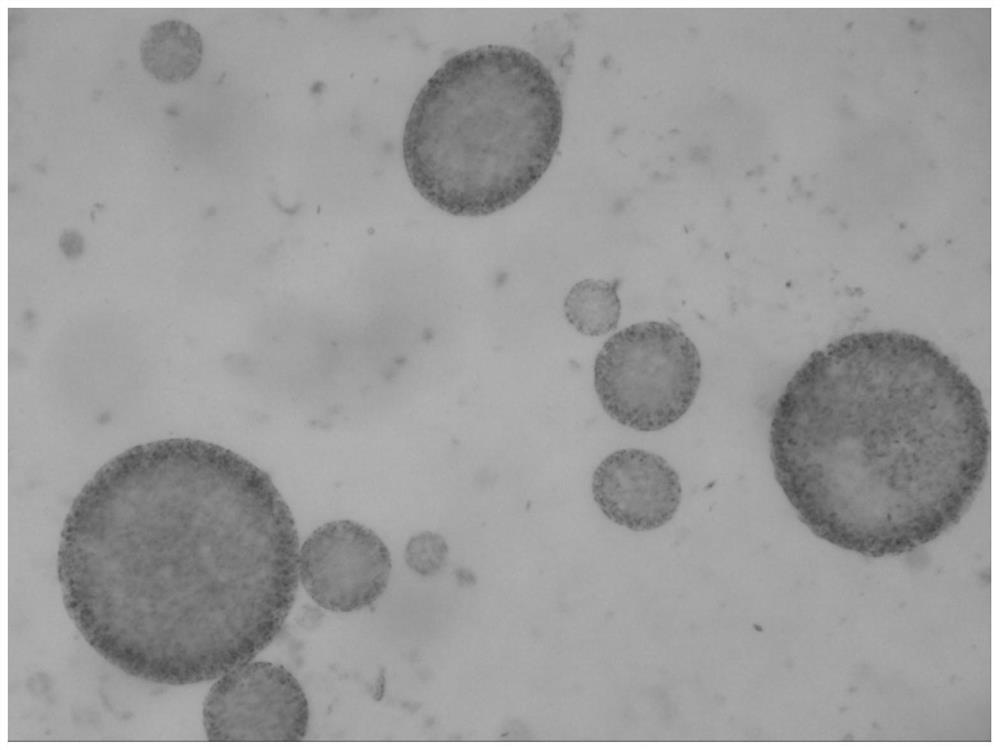
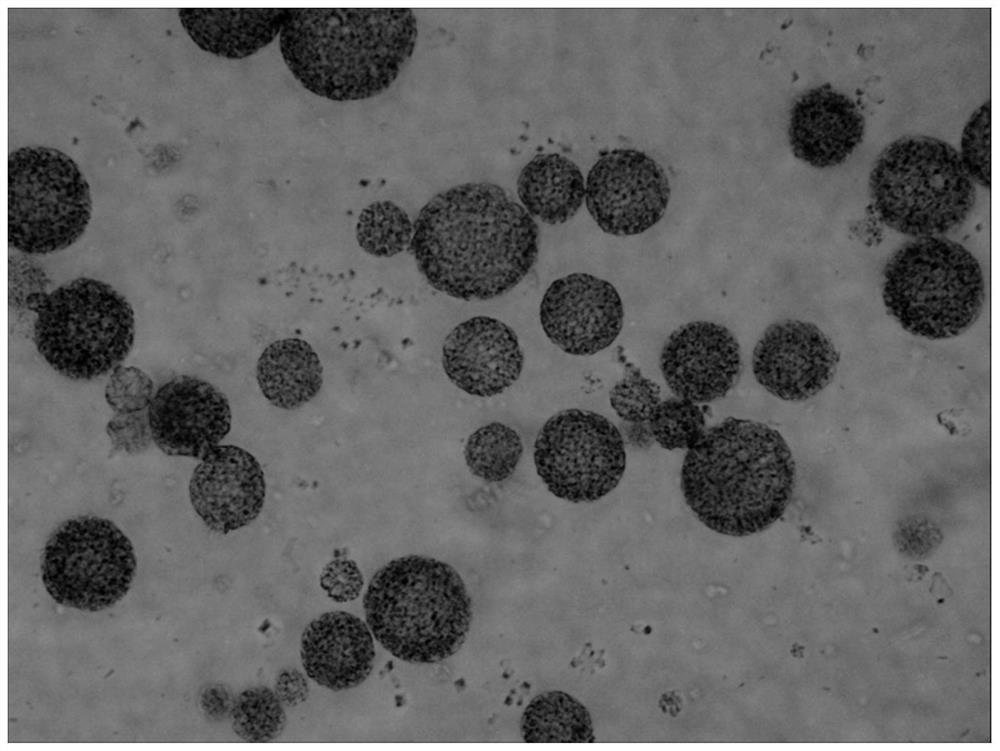
[0124] The preparation method of the bupivacaine multivesicular liposome provided in this example is basically the same as that in Example 1, except that in this example, the organic solvent of the oil phase is chloroform. The morphological observation results of the bupivacaine multivesicular liposomes prepared by the present embodiment under a microscope are as follows: Figure 8 shown.
[0125] Figure 8 The results showed that the multivesicular liposomes prepared in this example did not contain phospholipid fragments and were round in shape.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com