Injectable long-acting local anesthetic semi-solid gel formulations
A technology of semi-solid and pharmaceutical preparations, applied in the field of semi-solid gel preparations of injectable long-acting local anesthetics, which can solve the problems of unreliability, complicated and expensive manufacturing process, and poor repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0116] Preparation of castor oil semi-solid gel preparation
[0117] The castor oil semi-solid gel formulation containing active substances of the present invention can be prepared by directly mixing castor oil and gelling excipients, or by mixing with an already formed semi-solid gel matrix. The mechanical mixing process is carried out at a suitable temperature, usually between 60°C and 90°C, to completely melt the gelling excipient and castor oil into solution and dissolve or grind the active drug by any mechanical means to form a clear solution or homogeneous suspension. Vacuum can be applied to avoid air bubbles, and nitrogen can be applied to reduce oxidation of the active drug and delivery vehicle components. After obtaining a homogeneous pharmaceutical composition, the active substance semi-solid gel formulation can be cooled to room temperature.
[0118] Semi-solid gel drug composition of local anesthetic
[0119] Local anesthetics can cause temporary nerve conducti...
Embodiment 1
[0169] Example 1. SUP D
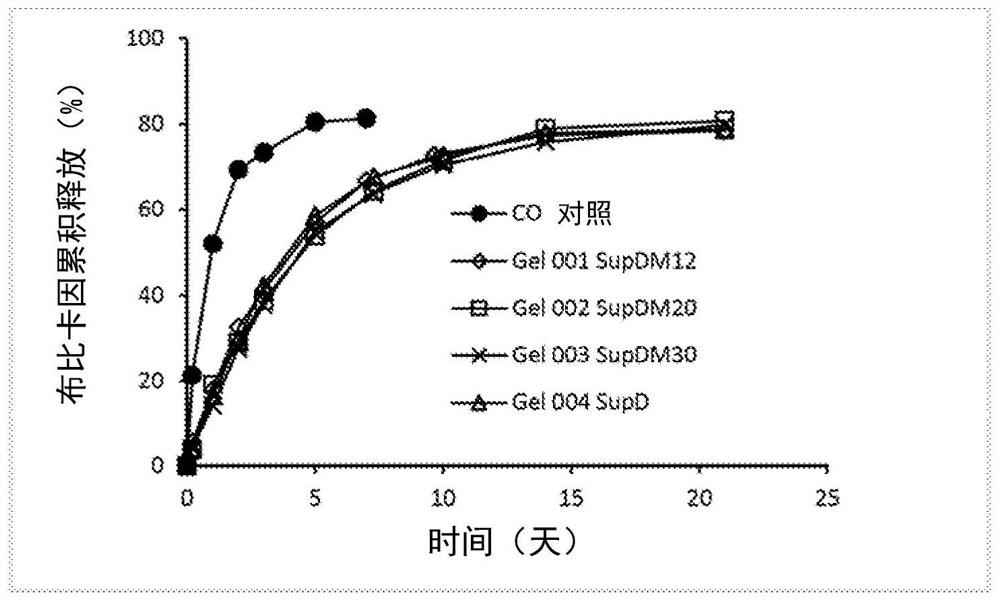
[0170] C 12 to C 18 SUP D mixture of triglycerides, melting point 42°C to 45°C. The results of the ratio study of castor oil and SUP D are shown in Table 3. Target amounts of each component were weighed into glass vials and heated to approximately 50°C. Place in 75°C water bath and mix / vortex until all components are completely dissolved and a clear solution forms.
[0171] SUP D takes about a similar time to start and complete gelation as SUP DM because they have similar properties and melting points. Approximately 1 mL of the hot solution was filled into a 5 mL prefilled syringe and steam sterilized at 121 °C for 20 min. Whether steam sterilized or not, after cooling to room temperature at 10-20 wt% gelling agent levels, they all appeared as homogeneous opaque gels and were injectable with a 21 gauge needle.
[0172] Table 3: Castor Oil and SUP D Ratio Study
[0173] Sample serial number Castor oil (g) SUP D(g) Bupivacaine (mg...
Embodiment 2
[0174] Example 2. SUP CM
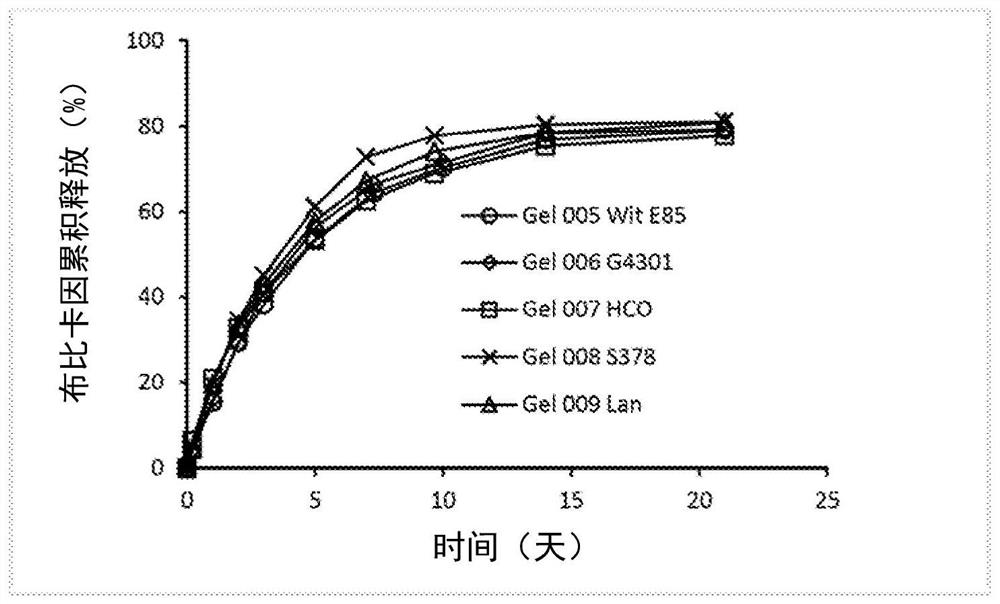
[0175] C 12 to C 18 The SUP CM mixture of triglycerides has a melting point of 37.8 to 39.8°C. Castor oil and SUP CM ratio studies are shown in Table 4. Target amounts of each component were weighed into glass vials and heated to approximately 75°C in a water bath and mixed / vortexed until all components were completely dissolved and a clear solution formed.
[0176] Since SUP CM has a lower melting point, SUP CM takes longer than SUP DM to initiate and complete gelation.
[0177] Approximately 1 mL of the hot solution was filled into a 5 mL prefilled syringe and steam sterilized at 121 °C for 20 min. Whether steam sterilized or not, after cooling to room temperature at 10-20 wt% gelling agent levels, they all appeared as homogeneous opaque gels and were injectable with a 21 gauge needle.
[0178] Table 4: Castor Oil and SUP CM Ratio Study
[0179] Sample serial number Castor oil (g) SUP CM(g) Bupivacaine (mg) SUP CM F01 1....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Water solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com