Method for simultaneously determining of concentration multi anesthesia medicament in blood plasma
A technology of local anesthesia and drug concentration, which is applied in the field of medical testing to reduce analysis costs, simplify analysis steps, and improve detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
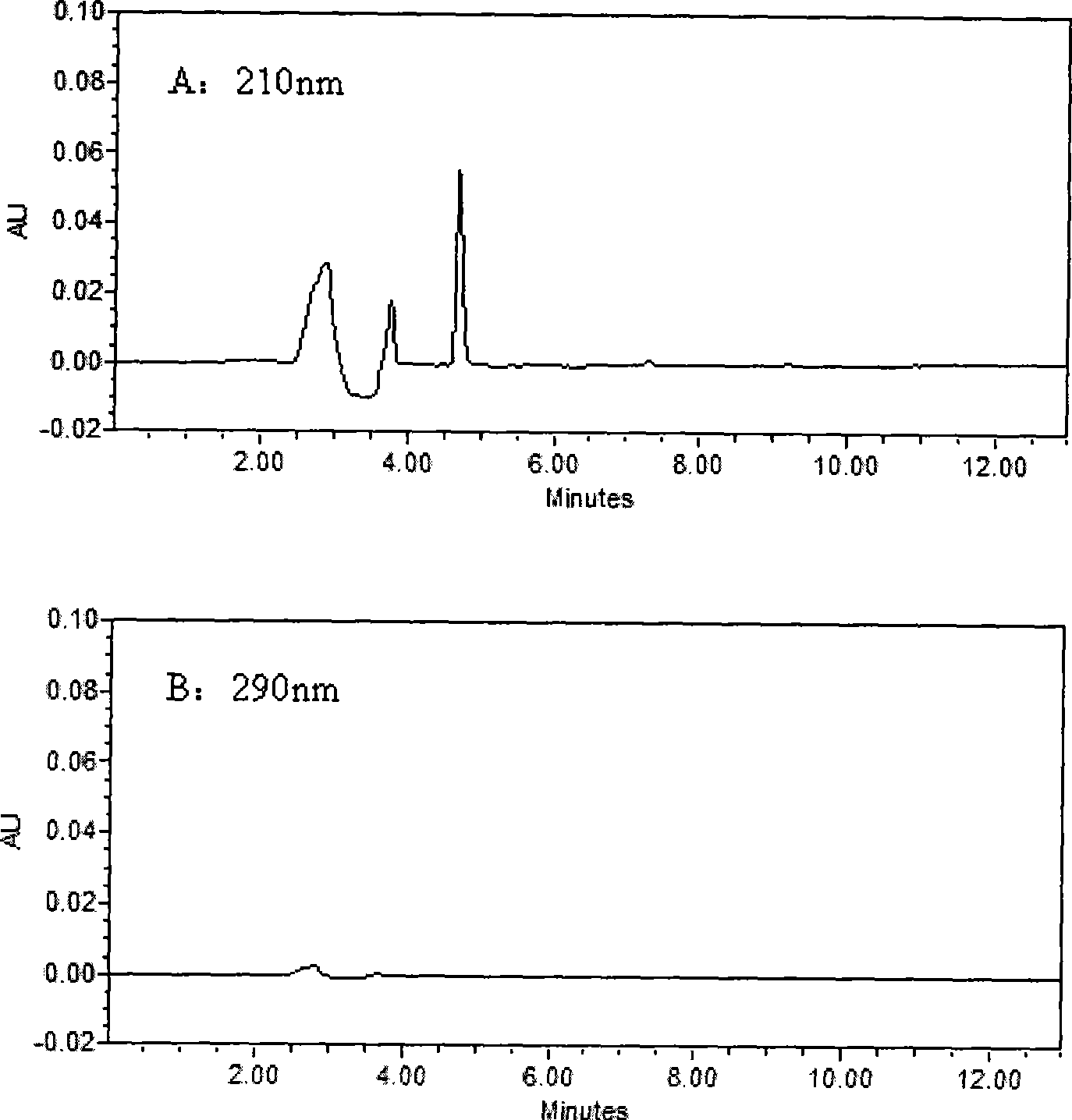
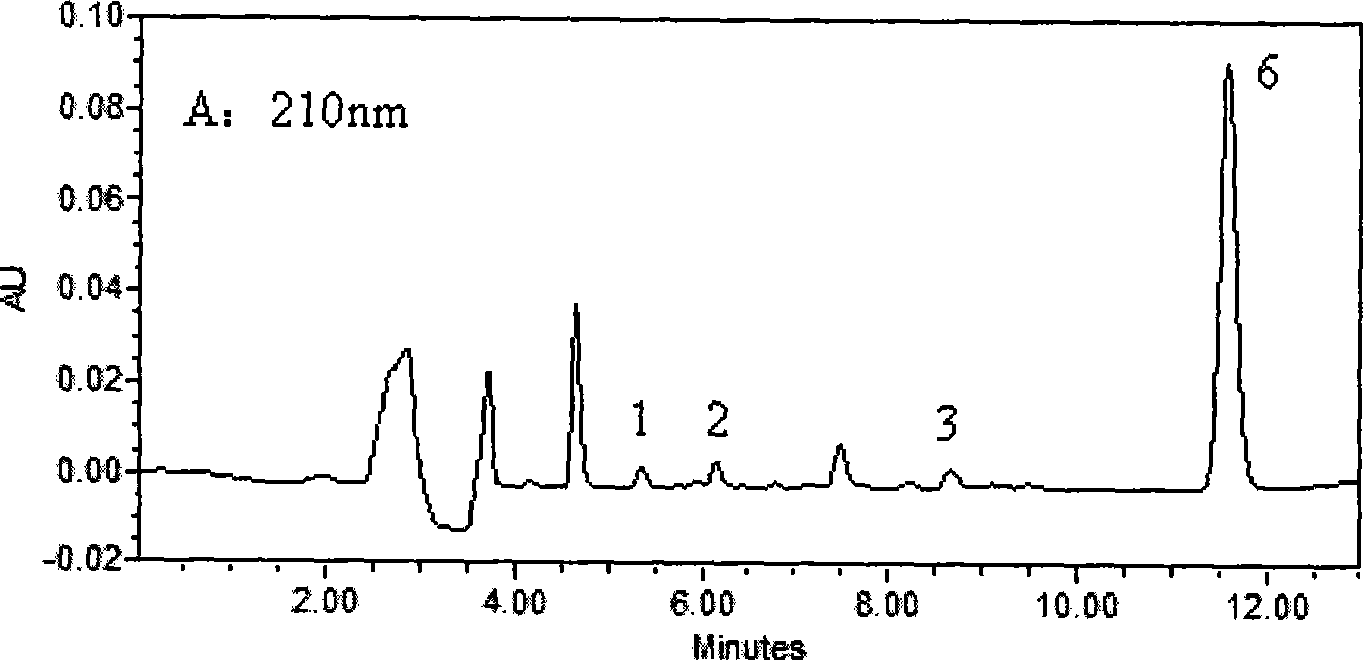
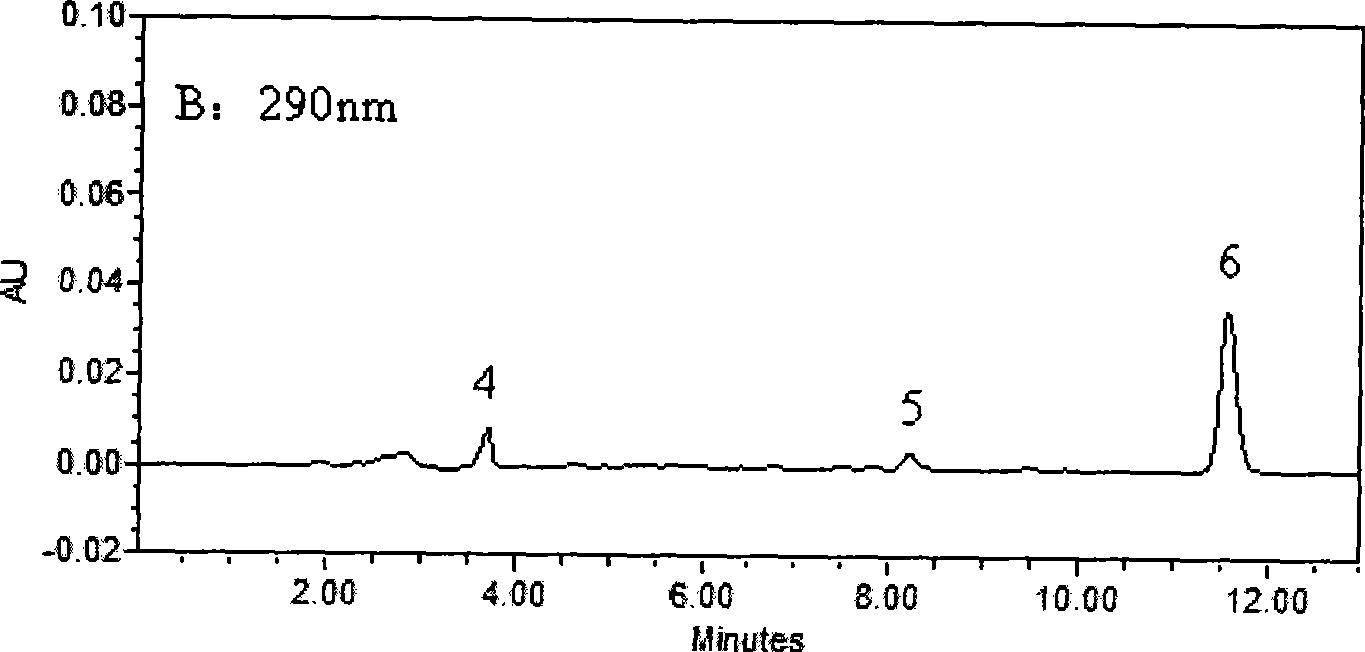
[0030] Chromatographic conditions
[0031] Waters 2690 HPLC system, Waters 2487 dual-wavelength UV detector, Millennium32 chromatographic workstation (Version 4.0); chromatographic column: Kromasil C 18 (250mm×4.6mm, 5μm); Column temperature: 40°C; Mobile phase: 30mmol / L potassium dihydrogen phosphate aqueous solution (containing 0.14% triethylamine solution, phosphoric acid solution to adjust pH=4.8): acetonitrile (61:39, V / V); flow rate 1.0mL min -1 ; The concentration of LID, ROP and BUP was measured at 210nm, and the concentration of PRO and TET was measured at 290nm.
[0032] Plasma sample pretreatment
[0033] Take 0.5mL blood sample and add 50μl of 0.5mg / mL neostigmine solution, 100μl of 5μg / mL internal standard solution and 100μL of 0.5mol / L KaOH solution, vortex for 10Sec, mix well, add 3mL of diethyl ether, all the above operations are at 3℃ Carry out under the following ice bath conditions; vortex for 2min, centrifuge at 2500g×8min, take 2.5mL of supernatant in ...
Embodiment 2
[0045] Chromatographic conditions
[0046] Waters 2690 HPLC system, Waters 2487 dual-wavelength UV detector, Millennium32 chromatographic workstation (Version 4.0); chromatographic column: Kromasil C 18 (250mm×4.6mm, 5μm); Column temperature: 40°C; Mobile phase: 30mmol / L potassium dihydrogen phosphate aqueous solution (containing 0.18% triethylamine solution, phosphoric acid solution to adjust pH=5.1): acetonitrile (65:35, V / V); flow rate 1.0mL min -1 ; The concentration of LID, ROP and BUP was measured at 210nm, and the concentration of PRO and TET was measured at 290nm.
[0047] Plasma sample pretreatment
[0048] Take 0.5mL blood sample and add 50μl of 2mg / mL neostigmine solution, 100μl of 5μg / mL internal standard solution and 100μL of 2mol / L NaOH solution, vortex for 8Sec, mix well, add 3mL of diethyl ether, the above operations are all under 3°C on ice Carry out under bath conditions; vortex for 2min, centrifuge at 2500g×10min, take 2.5mL supernatant in another test t...
Embodiment 3
[0059] Chromatographic conditions
[0060] Waters 2690 HPLC system, Waters 2487 dual-wavelength UV detector, Millennium32 chromatographic workstation (Version 4.0); chromatographic column: Kromasil C 18 (250mm×4.6mm, 5μm); Column temperature: 40°C; Mobile phase: 30mmol / L potassium dihydrogen phosphate aqueous solution (containing 0.16% triethylamine solution, phosphoric acid solution to adjust pH=4.9): acetonitrile (63:37, V / V); flow rate 1.0mL min -1 ; The concentration of LID, ROP and BUP was measured at 210nm, and the concentration of PRO and TET was measured at 290nm.
[0061] Plasma sample pretreatment
[0062] Take 0.5mL blood sample and add 50μl of 1mg / mL neostigmine solution, 100μl of 5μg / mL internal standard solution and 100μL of 1mol / L NaOH solution, vortex for 12Sec, mix well, add 3mL of diethyl ether, the above operations are all under 3°C on ice Under bath conditions; vortex for 2min, centrifuge at 3000g×9min, take 2.5mL supernatant in another test tube, blow ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com