Method for enriching piperidine-2-formanilide optically active compound
A formanilide and piperidine enrichment technology, applied in the direction of organic chemistry, can solve the problems of increased production of by-products, non-compliance with energy saving and consumption reduction, time-consuming crystallization process, etc., to achieve increased yield, reduced cost, reduced by-product effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The racemization and resolution of embodiment 1 intermediate
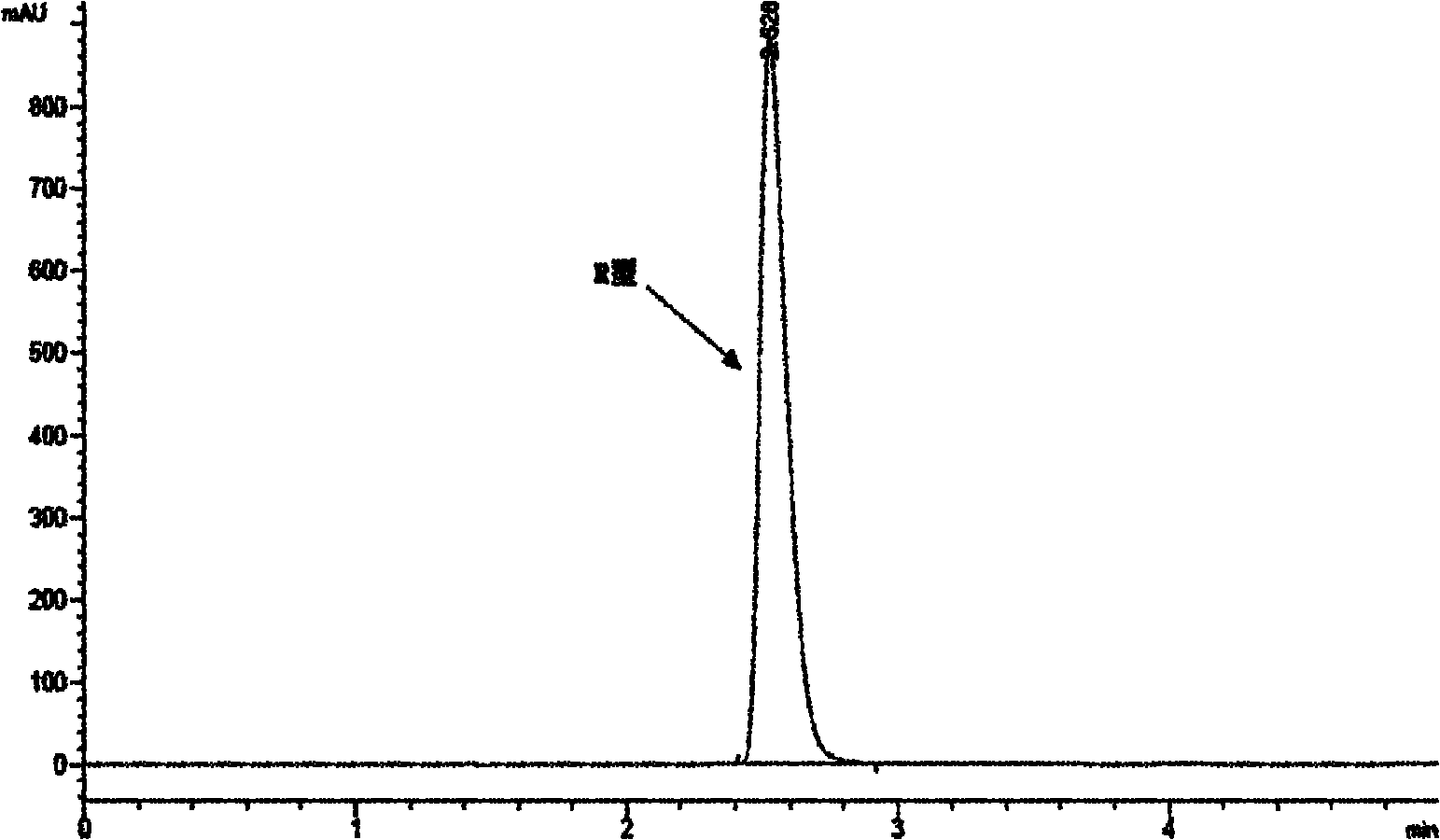
[0063] In 150ml toluene, add 17gR type intermediate (ee%=100, figure 1 ), 5ml of ethyl mercaptoacetate, and 5g of azobisisobutyronitrile were added to the racemization reaction system three times every 2 hours, and refluxed at 70°C for reaction.
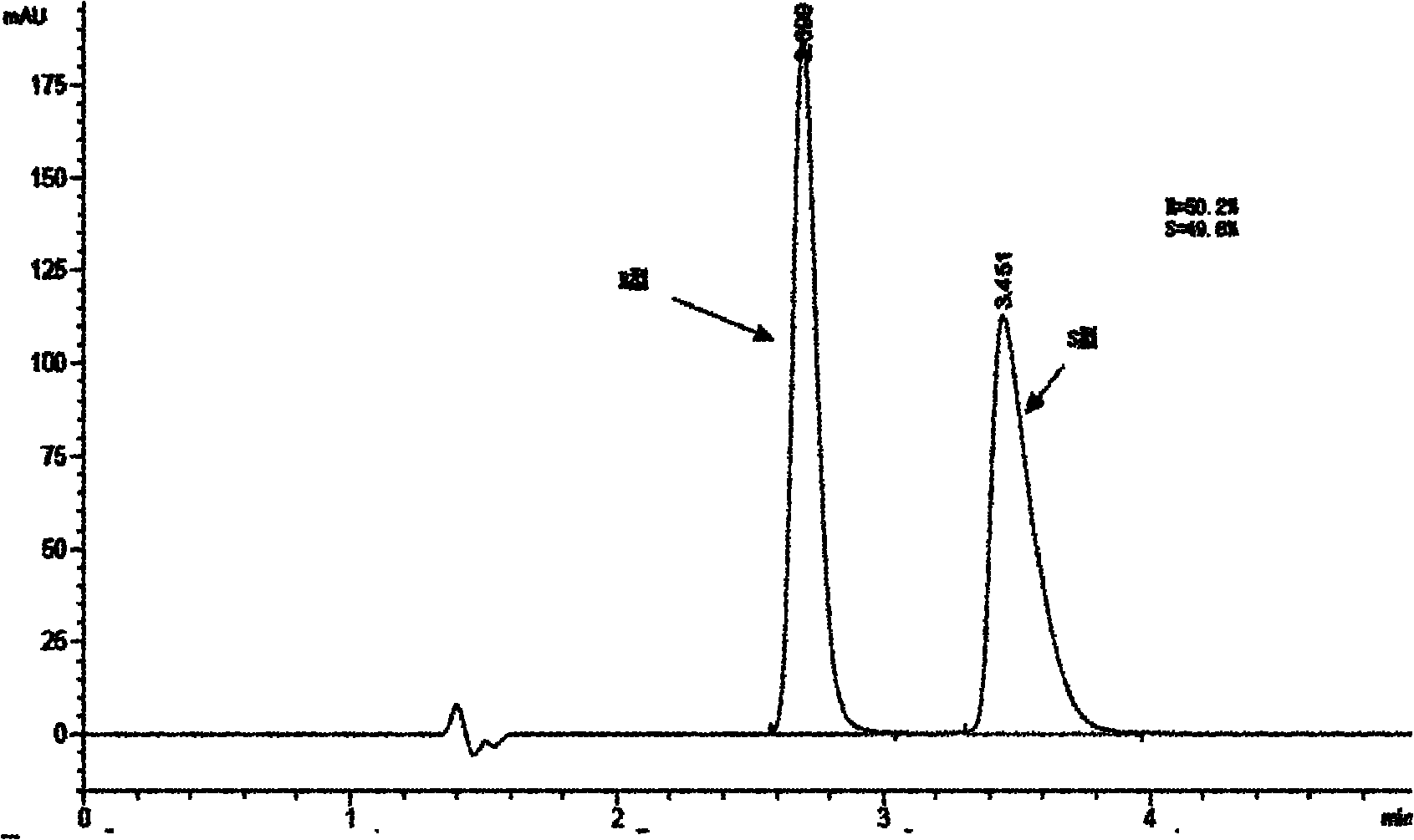
[0064] After reacting for 8 hours, add purified water to the reaction solution, adjust the pH value of the water layer to 4 with hydrochloric acid, extract with toluene, and separate the organic phase. The pH of the aqueous phase was adjusted to 10-11 with potassium hydroxide, extracted with toluene, the organic layer was dried over anhydrous sodium sulfate, and concentrated to obtain 15 g of racemic product (R=50.2%, S=49.8%, figure 2 ).
[0065] 15g of racemic intermediate pipecoloxylidide, and 11g of L-(-)-dibenzoyltartaric acid (DBTA) resolving agent were dissolved in 100ml of ethanol and stirred, then 35ml of acetone was added, and the separated white solid...
Embodiment 2
[0069] Embodiment 2 Racemization and resolution of ropivacaine
[0070] In 200ml toluene, add 10g ropivacaine (ee%=100, Figure 5 ), 3.1ml ethyl mercaptoacetate and 3.1g azobisisobutyronitrile were added to the racemization reaction system three times every 2 hours, and reflux reaction at 75-85°C.
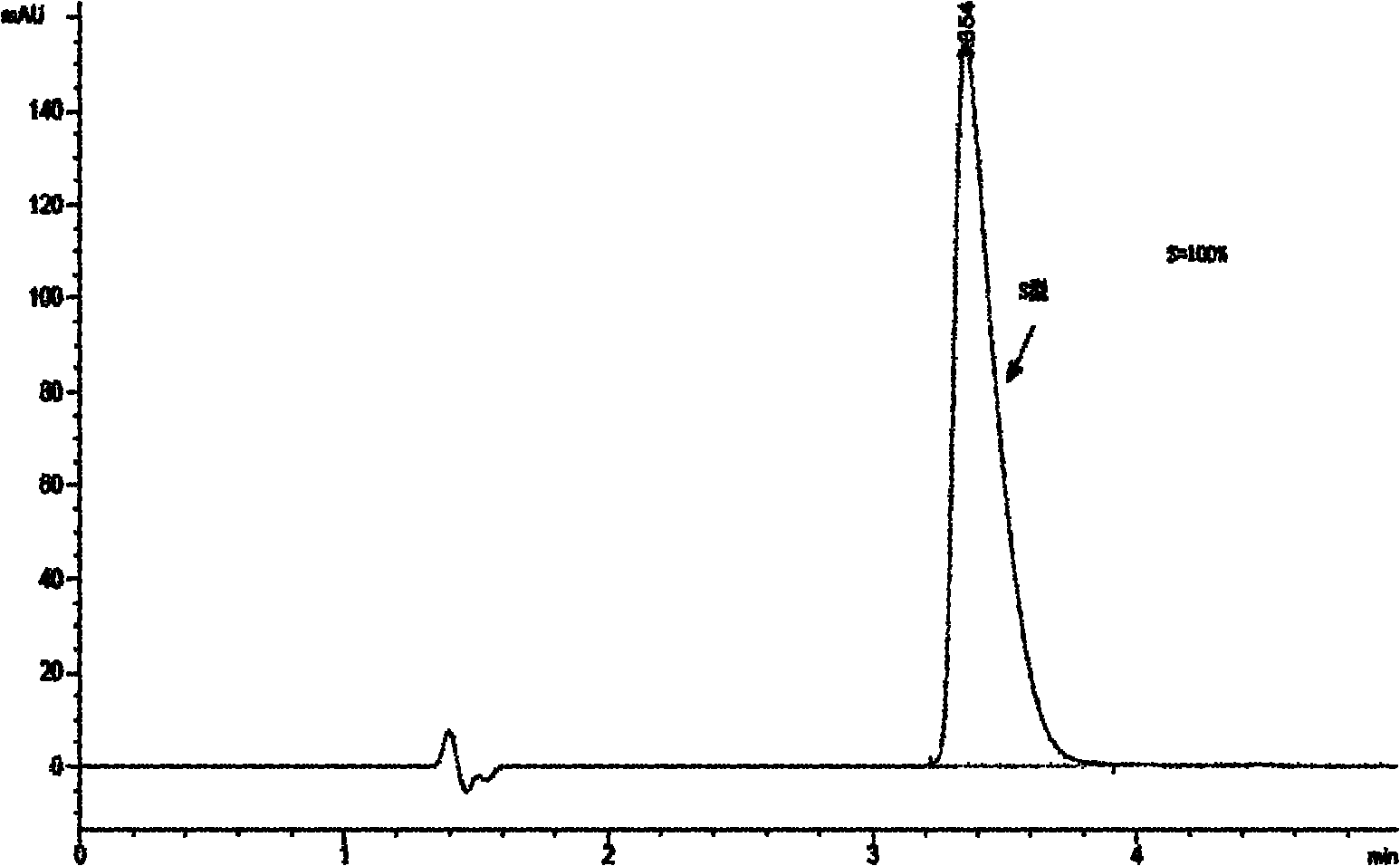
[0071] After reacting for 2 hours, add purified water to the reaction solution, adjust the pH value of the aqueous layer to 3-5 with hydrochloric acid, extract with p-xylene, and discard the organic phase. The aqueous phase was adjusted to a pH value of 9-11 with sodium hydroxide, extracted with p-xylene, and the organic layer was dried with anhydrous sodium sulfate and concentrated to obtain 8.7 g of a racemic product (ee%=0, Image 6 ).
[0072] 8.7g of racemic ropivacaine was dissolved in 45ml of n-butanol with 6.5g of L-(-)-dibenzoyltartaric acid (DBTA) resolving agent and stirred to form a salt. 19 ml of acetone was added, and the precipitated white solid was suction-filter...
Embodiment 3
[0076] Example 3 Racemization of Levobupivacaine
[0077] In 300ml toluene, add 15g levobupivacaine (ee%=100, Figure 9 ), 4.6ml of ethyl mercaptoacetate and 4.6g of azobisisobutyronitrile were added to the reaction system three times every 2h, and the reaction was refluxed at 85-95°C.
[0078] After reacting for 10 hours, add purified water to the reaction solution, adjust the pH of the aqueous layer to 4-5 with hydrochloric acid, extract with o-xylene, and discard the organic phase. The aqueous phase was adjusted to a pH of 10 to 11 with sodium hydroxide, extracted with ortho-xylene, the organic layer was dried with anhydrous sodium sulfate, and concentrated to obtain 12.3 g of racemic product (ee%=0, Figure 10 )
[0079] 12.3 g of racemic levobupivacaine, and 9.18 g of L-(-)-dibenzoyltartaric acid (DBTA) resolving agent were dissolved in 35 ml of methanol and stirred to form a salt. 15ml of acetone was added, and the precipitated white solid was suction-filtered to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com