Patents
Literature
37 results about "Chondroitin sulphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Treatment of central nervous system damage
InactiveUS20050118157A1Decreased inhibitory propertiesImprove plasticityCompound screeningNervous disorderNervous systemChondroitinase ABC
The invention provides a method of promoting neuronal plasticity in the CNS of a mammal, the method comprising administering to the CNS of the mammal an agent that reduces the inhibitory properties of chondroitin sulphate proteoglycans. Preferred agents are chondroitinases and sulfatases, e.g., chondroitinase ABC. Also provided are methods of identifying further agents.
Owner:KING'S COLLEGE LONDON +1
Method of Improving Treatments in Rheumatic and Arthritic Diseases
Improved treatments of joint diseases, such as, e.g. osteoarthritis and rheumatoid arthritis, and pain, wherein a strontium-containing compound is administered alone or in combination with one or more second therapeutically and / or prophylactically active substances, selected from the group consisting of bisphosphonates, glucosamine, pallitative agents, analgesic agents, disease modifying anti-rheumatic compounds (DMARDs), selective estrogen receptor modulators (SERMs), aromatase inhibitors, non-steroidal anti-inflammatory agents (NSAIDs), COX-2 inhibitors, COX-3 inhibitors, opioids, inhibitors / antagonists of IL-1, inhibitors / antagonists of TNF-alpha, inhibitors of matrix metallo-proteinases (MMPs), cathepsin K inhibitors, inhibitors / antagonists of RANK-ligand, statins, glucocorticoids, chondroitin sulphate, NMDA receptor antagonists, inhibitors of interleukin-I converting enzyme, Calcitonin gene related peptide antagonists, glycine antagonists, vanilloid receptor antagonists, inhibitors of inducible nitric oxide synthetase (iNOS), N-acetylcholine receptor agonists, neurokinin antagonists, neuroleptic agents, PAR2 receptor antagonists and anabolic growth factors acting on joint tissue components. Pharmaceutical compositions comprising a strontium-containing compound and a second therapeutically and / or prophylactically active substance as defined above.
Owner:OSTEOLOGIX AS
Method and pharmaceutical to treat spinal discs
Methods for reducing chronic pain caused by a disrupted spinal disc are described. In one method, a physiologically acceptable amount of an injectable is injected into the disc. The injectable is obtained from a stock solution comprising chondroitin sulphate, glucosamine HCl, aqueous solution of dextrose; sodium carboxymethylcellulose, and a buffer substance in quantity to bring the pH of the stock solution to a value above about 6.0. Water is also added to dilute the stock solution. The stock solution may further comprise an anesthetic such as bupivicaine.
Owner:NOTOGEN INC
Injectable bone regeneration gel containing bone formation enhancing peptide
ActiveUS20100317587A1Peptide/protein ingredientsPharmaceutical delivery mechanismBone Marrow Stromal CellTreatment effect
The present invention relates to an injectable bone regeneration material containing a bone formation enhancing peptide, and more particularly, to an injectable bone regeneration material, in which a bone formation enhancing peptide essentially containing one and more amino acid sequences among SEQ ID NO: 1 to SEQ ID NO: 28 is bonded or mixed to a gel-forming base material selected from the group consisting of chitosan, alginic acid, silk fibroin, propylene glycol, propylene glycol alginic acid, poloxamer, chondroitin sulphate, and the combination thereof. The injectable bone regeneration material according to the present invention can increase differentiation of bone marrow stromal cells and osteoblasts into bone tissue, thus maximizing tissue regeneration by a peptide capable of promoting differentiation of bone tissue and periodontal tissue regeneration. The injectable bone regeneration material is in the form of a gel, and thus can be applied to a surface of various medical devices such as implant etc., and can be mixed with bone graft particles to apply, so that it can increase a treatment effect of existing medical devices to maximize a tissue regeneration effect.
Owner:NANO INTELLIGENT BIOMEDICAL ENG +1
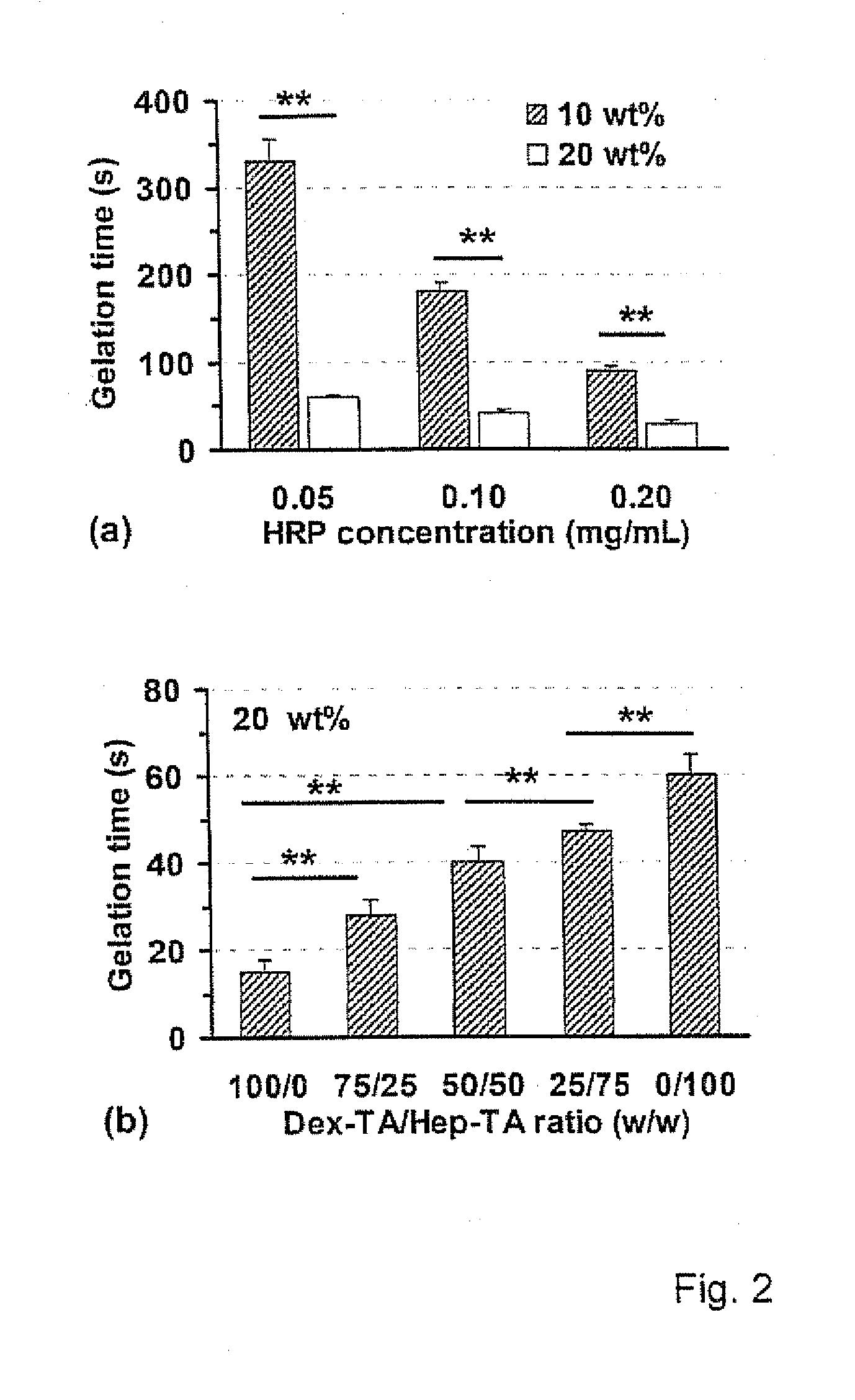
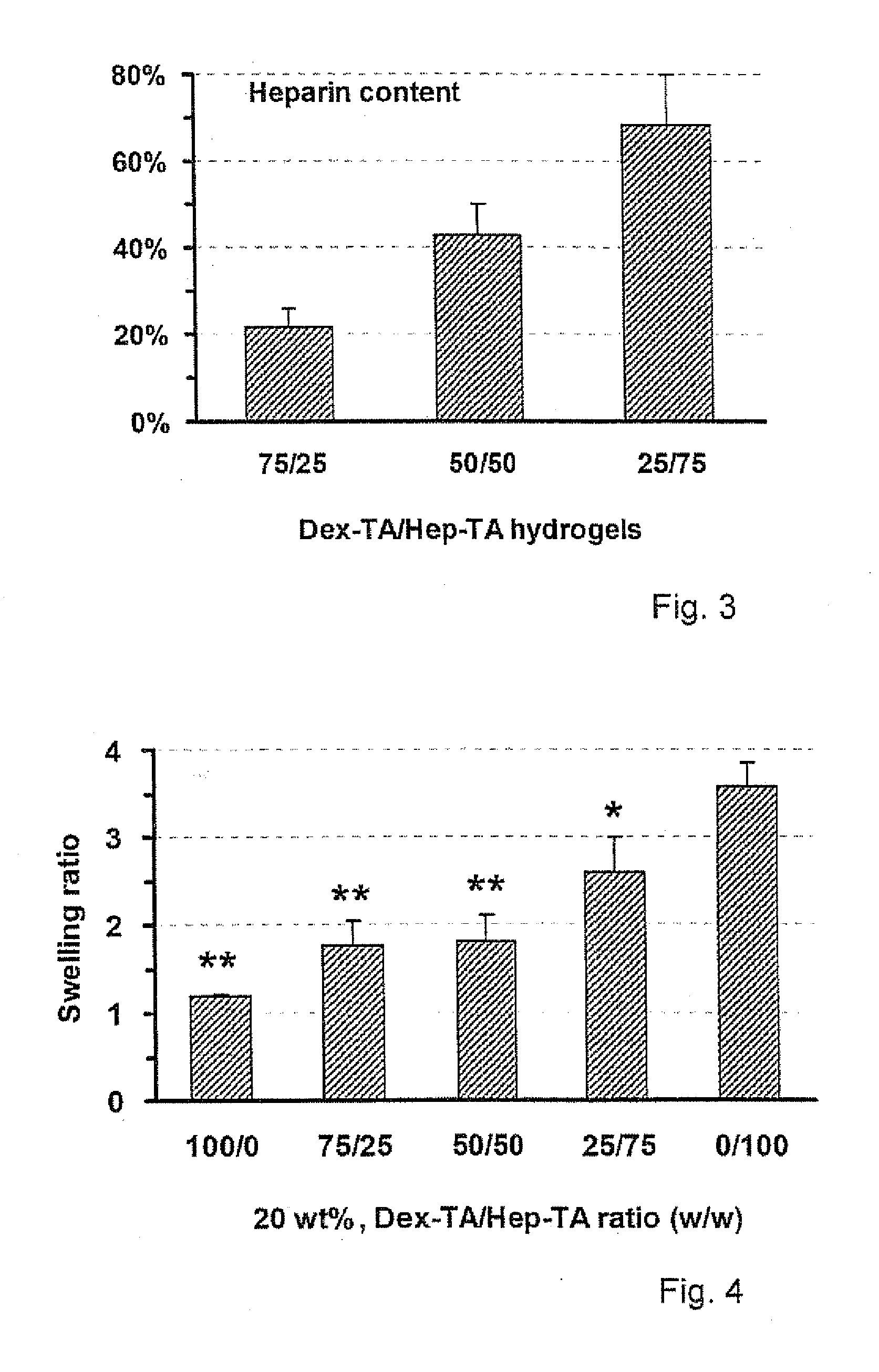
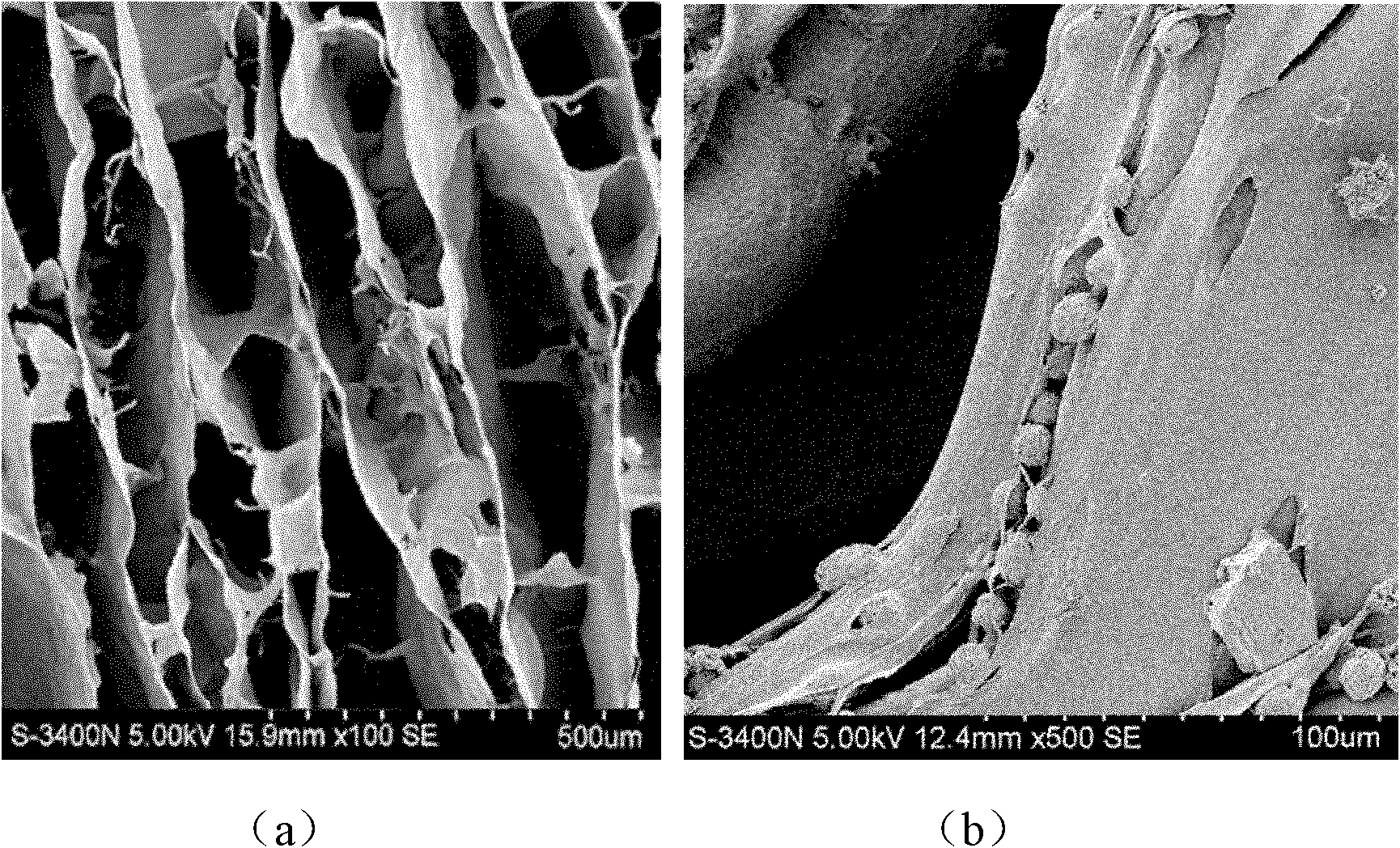
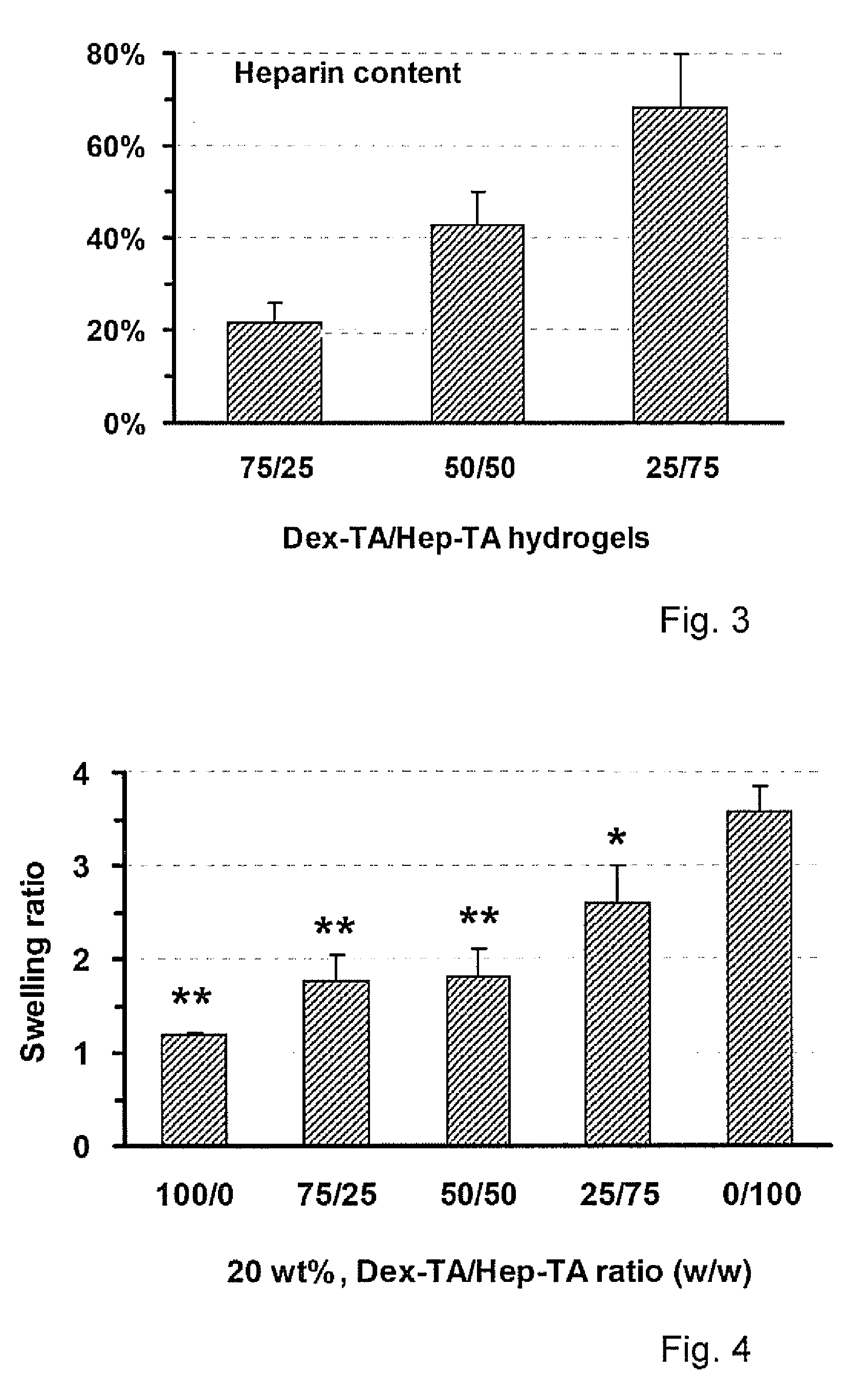
Hydrogels based on polymers of dextran tyramine and tyramine conjugates of natural polymers
The invention relates to composition comprising a dextran-tyramine conjugate and a conjugate selected from the group consisting of chondroitin sulphate-tyramine, collagen-tyramine, chitosan-tyramine, chitosan-phloretic acid, gelatine-tyramine, heparan sulphate-tyramine, keratin sulphate-tyramine, hyaluronic acid-tyramine and heparin-tyramine.
Owner:HY2CARE BV
Cartilage deficiency prosthesis, preparation method thereof and integrated cartilage-bone repair material
InactiveCN101954121AGood biocompatibilityStable mechanical strengthProsthesisMass ratioArticular cartilage injuries
The invention discloses cartilage deficiency prosthesis, a preparation method thereof and an integrated cartilage-bone repair material. The prosthesis is composed of collagen and proteoglycan based on the mass ratio of 1 / 3-3, wherein, the collagen is I-type collagen or II-type collagen, and the proteoglycan comprises glycosaminoglycan, chitosan or chondroitin sulfate; and natural cartilage matrixes are longitudinally arranged in the prosthesis. The integrated cartilage-bone repair material is made by compounding the prosthesis with a bone matrix material together. The cartilage defect prosthesis of an articular cartilage matrix structure of the invention can meet the repair need of articular cartilage deficiency, effectively improve repair rate of articular cartilage injury and lower disability incidence of a patient.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Injectable compositions for intra-articular use combining a viscosupplementation agent and a fibroblast growth medium
InactiveUS20120237610A1Extend your lifeIncrease battery capacityBiocideOrganic active ingredientsCelluloseIntra-articular use
Provided is an injectable composition for intra-articular administration including at least one viscosupplementation agent selected from hyaluronic acid, chondroitin sulphate, keratin, keratin sulphate, heparin, cellulose and derivatives thereof, for example, chitosan, xanthans, galactomannan, alginates, and one or more salts thereof, and a fibroblast growth medium. The viscosupplementation agent and the fibroblast growth medium can be provided in a single composition for injection or as separate components for simultaneous, separate, or subsequent injection over time.
Owner:THOREL JEAN NOEL
Hydrogels based on polymers of dextran tyramine and tyramine conjugates of natural polymers
The invention relates to composition comprising a dextran-tyramine conjugate and a conjugate selected from the group consisting of chondroitin sulphate-tyramine, collagen-tyramine, chitosan-tyramine, chitosan-phloretic acid, gelatine-tyramine, heparan sulphate-tyramine, keratin sulphate-tyramine, hyaluronic acid-tyramine and heparin-tyramine.
Owner:HY2CARE BV
Compositions and methods for altering elastogenesis
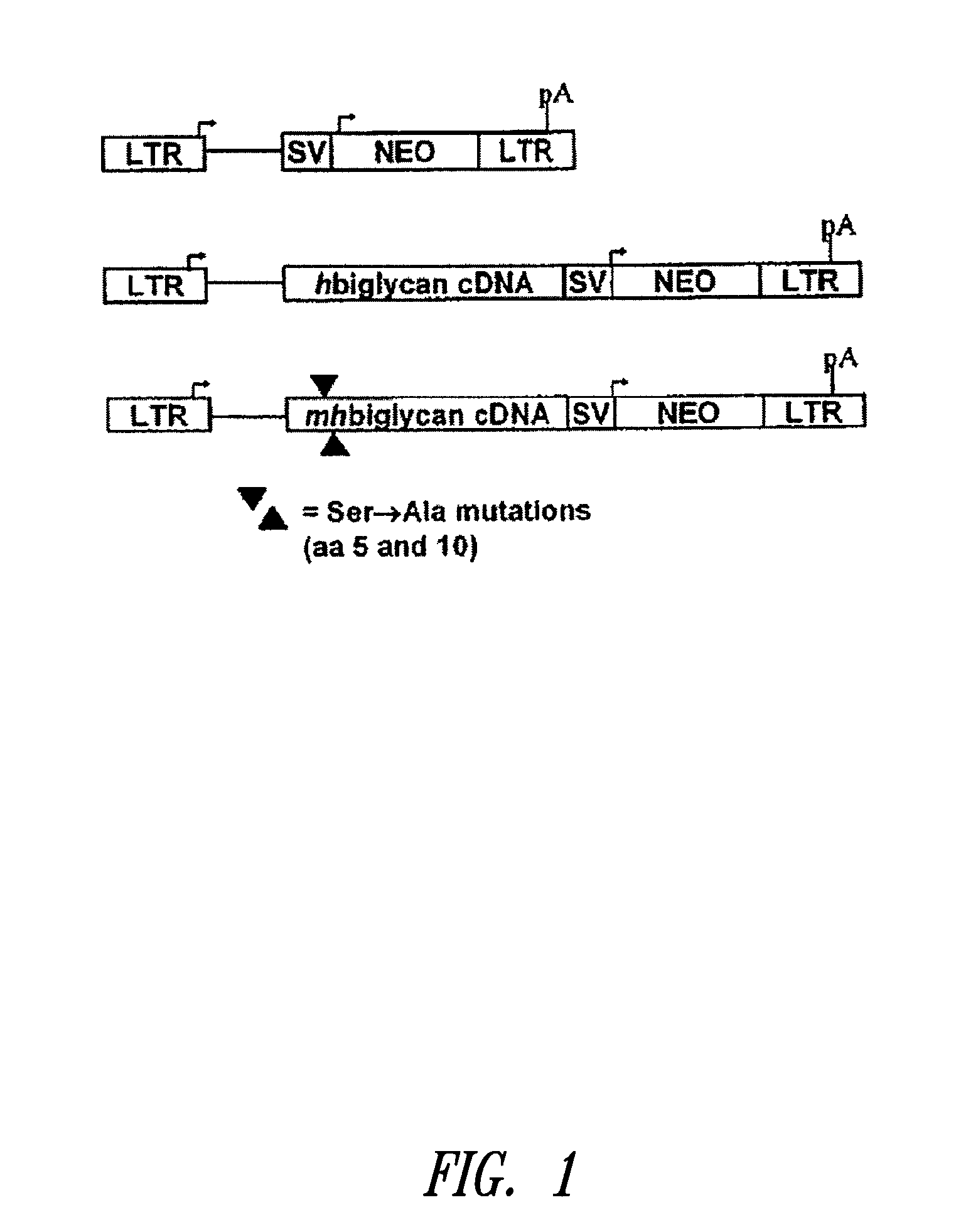
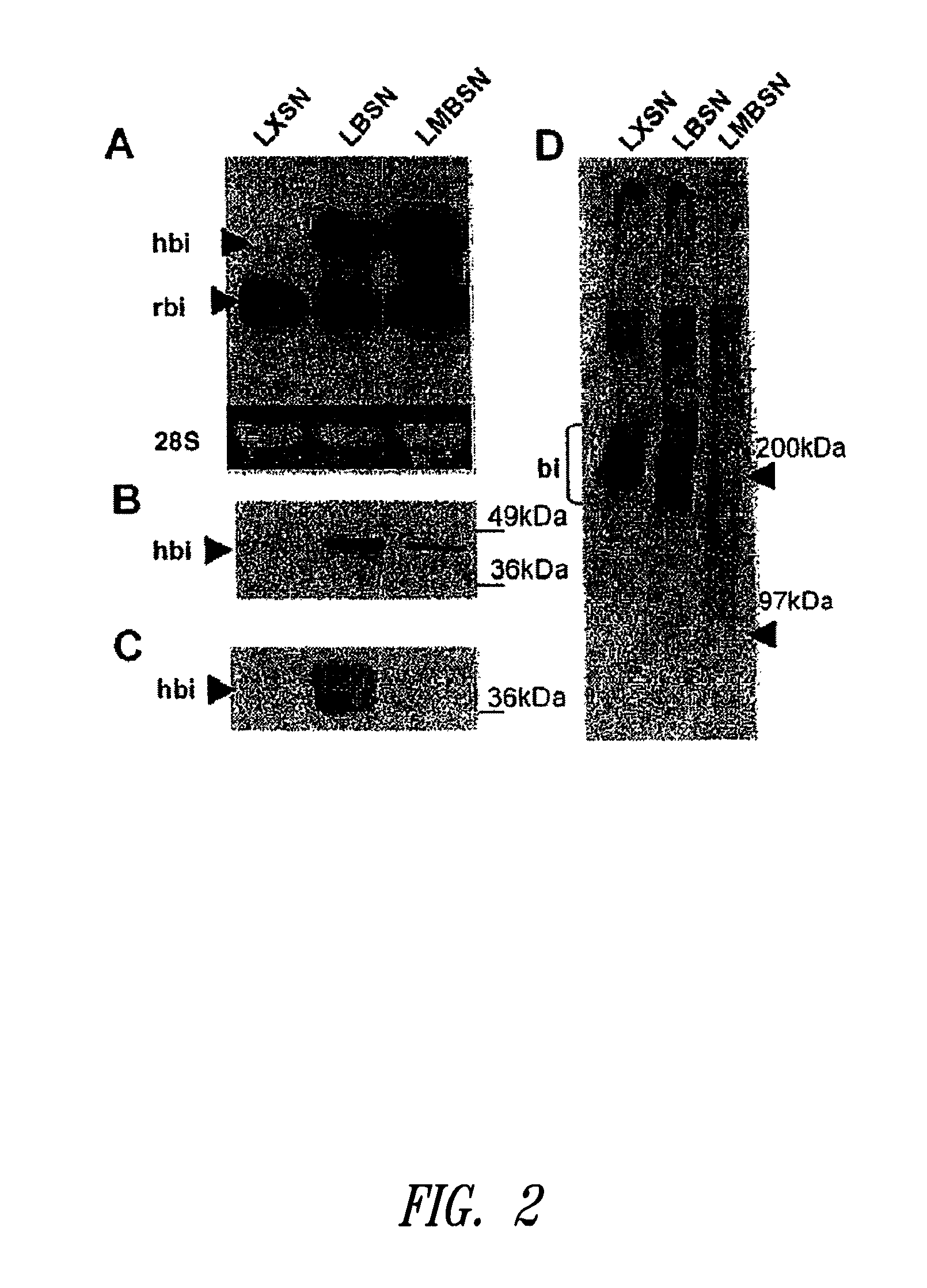
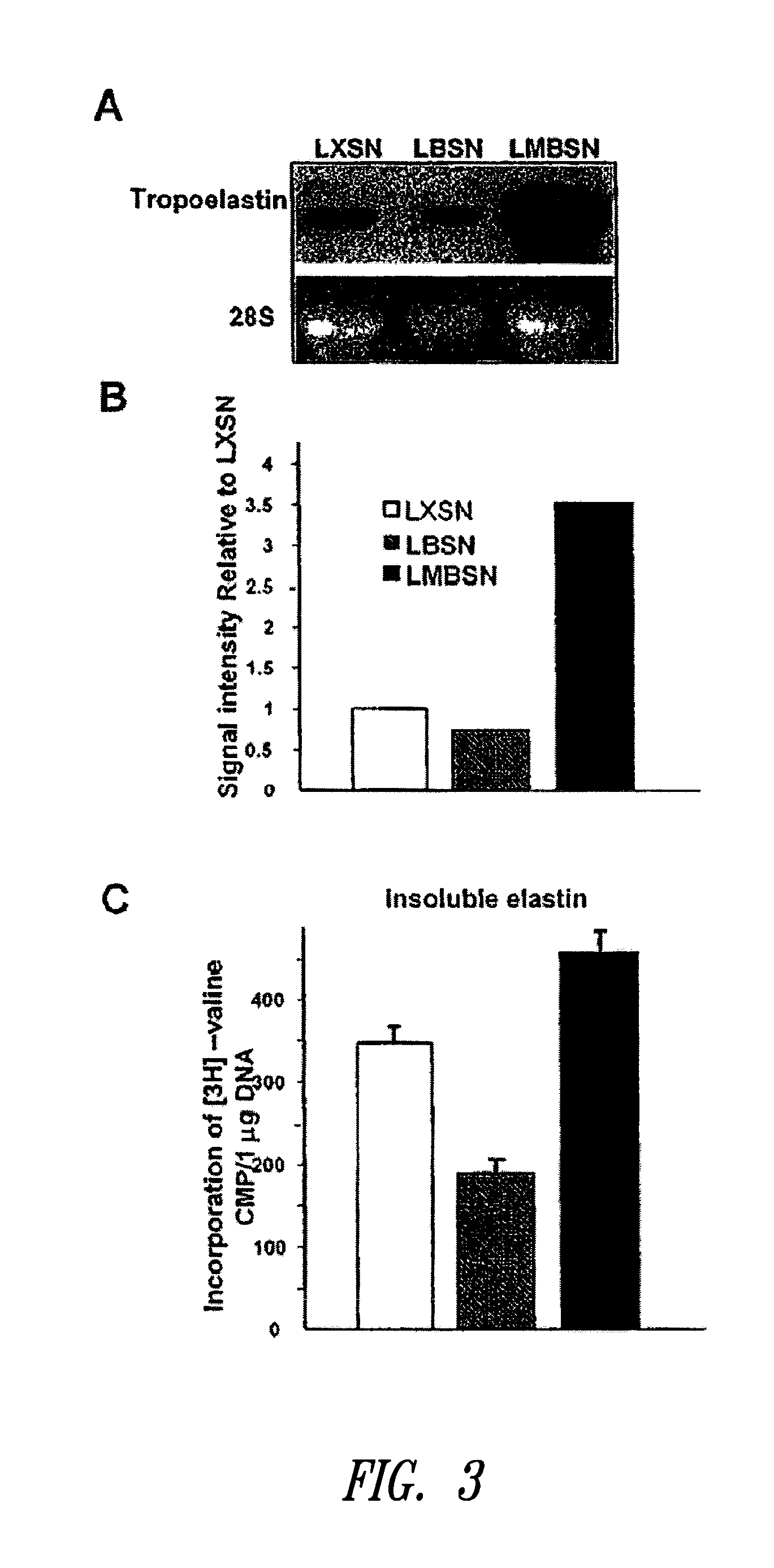
InactiveUS20100197563A1Promoting elastogenesisOrganic active ingredientsPeptide/protein ingredientsChondroitin sulphateBiglycan
Compositions and methods are provided for promoting elastin fiber formation (elastogenesis) in a cell, including methods that comprise contacting a cell that is capable of elastogenesis with (i) a mutated biglycan polypeptide that lacks chondroitin sulphate proteoglycan chains, (ii) a versican V3 isoform polypeptide that lacks most or all of the polypeptide regions encoded by one or more of exons 4, 5 or 6 or by exons 9-10 or 11-13, and / or with (iii) metastatin.
Owner:BENAROYA RES INST AT VIRGINIA MASON
Injectable bone regeneration gel containing bone formation enhancing peptide
ActiveUS8546529B2Peptide/protein ingredientsPharmaceutical delivery mechanismBone Marrow Stromal CellOsteoblast
The present invention relates to an injectable bone regeneration material containing a bone formation enhancing peptide, and more particularly, to an injectable bone regeneration material, in which a bone formation enhancing peptide essentially containing one and more amino acid sequences among SEQ ID NO: 1 to SEQ ID NO: 28 is bonded or mixed to a gel-forming base material selected from the group consisting of chitosan, alginic acid, silk fibroin, propylene glycol, propylene glycol alginic acid, poloxamer, chondroitin sulphate, and the combination thereof. The injectable bone regeneration material according to the present invention can increase differentiation of bone marrow stromal cells and osteoblasts into bone tissue, thus maximizing tissue regeneration by a peptide capable of promoting differentiation of bone tissue and periodontal tissue regeneration. The injectable bone regeneration material is in the form of a gel, and thus can be applied to a surface of various medical devices such as implant etc., and can be mixed with bone graft particles to apply, so that it can increase a treatment effect of existing medical devices to maximize a tissue regeneration effect.
Owner:NANO INTELLIGENT BIOMEDICAL ENG +1
Compounds having magnetic functionality, implants or gels derived from same, and use of both in order to determine the enzyme activity of an enzyme
InactiveUS20150174272A1Measurable variation in its magnetic propertyMeasurable variation in its magnetic propertiesPeptide/protein ingredientsDisease diagnosisCell-Extracellular MatrixChondroitin sulphate
The invention relates to an implant or gel including at least one compound having magnetic functionality, which in turn includes a substrate of at least one enzyme involved in regenerating the extracellular matrix and a plurality of magnetic particles. The invention also relates to a compound having magnetic functionality which includes a substrate of at least one enzyme involved in regenerating the extracellular matrix and a plurality of magnetic particles, preferably with no coating or surface modification, or functionalised with carboxyl groups, for the production of an implant or gel for monitoring an enzyme activity involved in regenerating the extracellular matrix. The substrate is preferably selected from the group that comprises collagen, chondroitin sulphate and hyaluronic acid, and the magnetic particles are superparamagnetic particles.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC)
Corneal metaphase preserving liquid containing recombinant human serum albumin and preparation method thereof
InactiveCN109329274AMaintain structureMaintain transparencyDead animal preservationSodium bicarbonateGlutamine
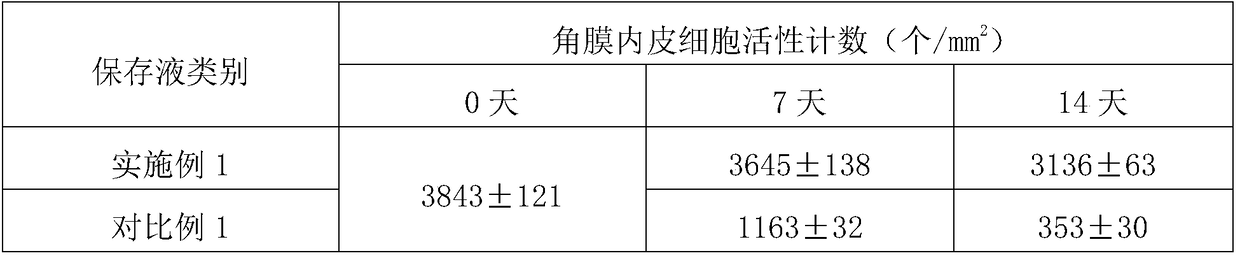
The invention discloses corneal metaphase preserving liquid containing recombinant human serum albumin and a preparation method thereof. The corneal metaphase preserving liquid containing recombinanthuman serum albumin contains glutamine, calcium pantothenate, choline chloride, folic acid, inositol, nicotinamide, pyridoxal.HCl, riboflavin, thiamine.HCl, KCl, NaCl, NaH2PO4.2H2O, glucose, dextran 40, sodium pyruvate, vitamin C, chondroitin sulphate sodium, sodium hyaluronate, non-animal recombinant human serum albumin, gentamicin sulfate, sodium bicarbonate, piperazine-1-erhanesulfonic acid, phenol red indicator, and water for injection. The non-animal recombinant human serum albumin is added to replace serum to improve corneal endothelial cell survival rate; and then the non-animal recombinant human serum albumin cooperates with a compound system of the chondroitin sulphate sodium, the sodium hyaluronate and the dextran 40, the survival rate of the corneal endothelial cell is greatly ensured.
Owner:镇江雷音再生医学科技有限公司
Compounds useful in the diagnosis and treatment of pregnancy-associated malaria
ActiveUS7745580B2Raise the possibilitySimple processPeptide/protein ingredientsProtozoaNucleotideSpecific igg
The present invention relates to nucleic acid molecules related to the var2csa gene family as well as amino acid sequences encoded by such nucleic acid molecules with respect to their role in mediating adhesion of infected red blood cells to chondroitin sulphate A (CSA) in the placenta which is characteristic for the pathogenesis of pregnancy associated malaria (PAM). Accordingly, The invention provides the use compounds that are related to VAR2CSA polypeptides var2csa nucleic acid molecules as medicaments, as well as it provides pharmaceutical compositions, in particular immunological compositions and vaccines, hereunder nucleotide-based vaccines comprising these compounds. In addition, the invention provides the use of the compounds mentioned for the manufacture of compositions, such as immunogenic compositions. Other aspects of the invention relates to methods of treatment and prevention of pregnancy associated malaria wherein these methods are based on the nucleic acid molecules and polypeptides the invention. As these compounds can also be used as biotechnological tools the invention provides in vitro diagnostic methods and kits comprising reagents and IgGs / antibodies designated to the use in such methods. The invention also relates to methods of identifying agents capable of modulating the VAR2CSA dependent adhesion to CSA and agent capable of interacting with VAR2CSA. Finally, a method for identifying polypeptides, which will induce a specific IgG / antibody response upon administration to a subject is provided by the invention.
Owner:UNIVERSITY OF COPENHAGEN
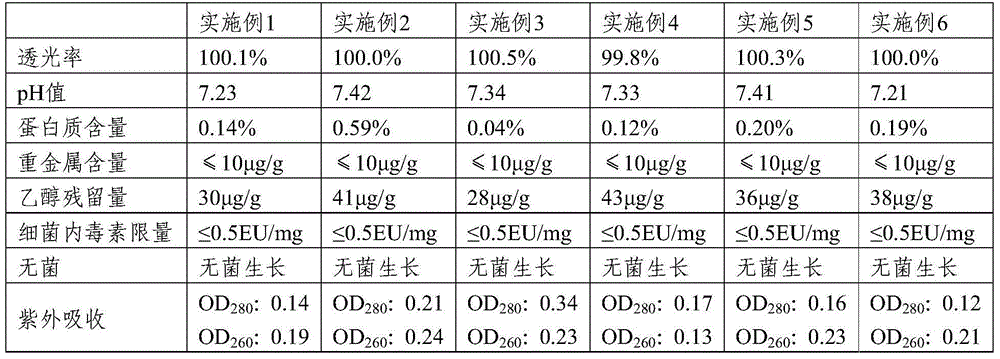
Method fo preparing medium, low molecular weight chondroitin sulphate and obtained product
A medium (or low)-molecular sulfate chondroitin is prepared through acid hydrolysis, alkali neutralization, separation, ultrafiltering, nanofiltering, and spray drying or crystallizing in alcohol. Its advnatages are high purity and easy absorption.
Owner:汤毅
Methods for promoting elastogenesis and elastin fiber formation by increasing tropoelastin expression
InactiveUS8367619B2Promoting elastogenesisPeptide/protein ingredientsSkeletal disorderChondroitin sulphateExon
Compositions and methods are provided for promoting elastin fiber formation (elastogenesis) in a cell, including methods that comprise contacting a cell that is capable of elastogenesis with (i) a mutated biglycan polypeptide that lacks chondroitin sulphate proteoglycan chains, (ii) a versican V3 isoform polypeptide that lacks most or all of the polypeptide regions encoded by one or more of exons 4, 5 or 6 or by exons 9-10 or 11-13, and / or with (iii) metastatin.
Owner:BENAROYA RES INST AT VIRGINIA MASON
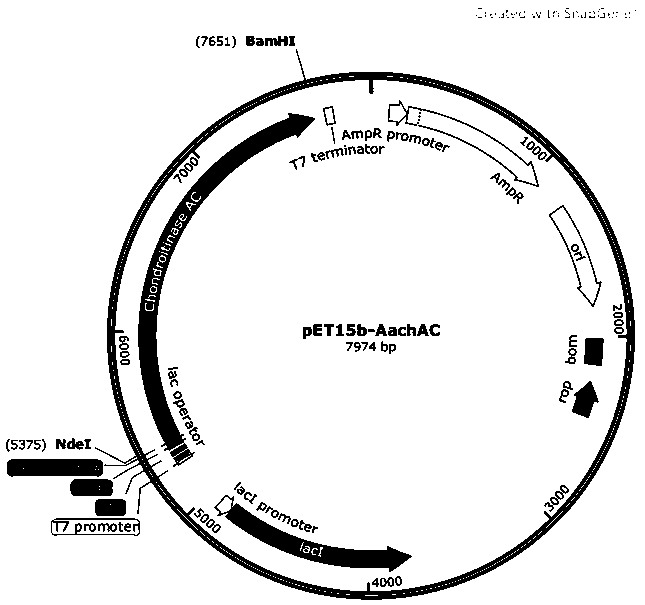
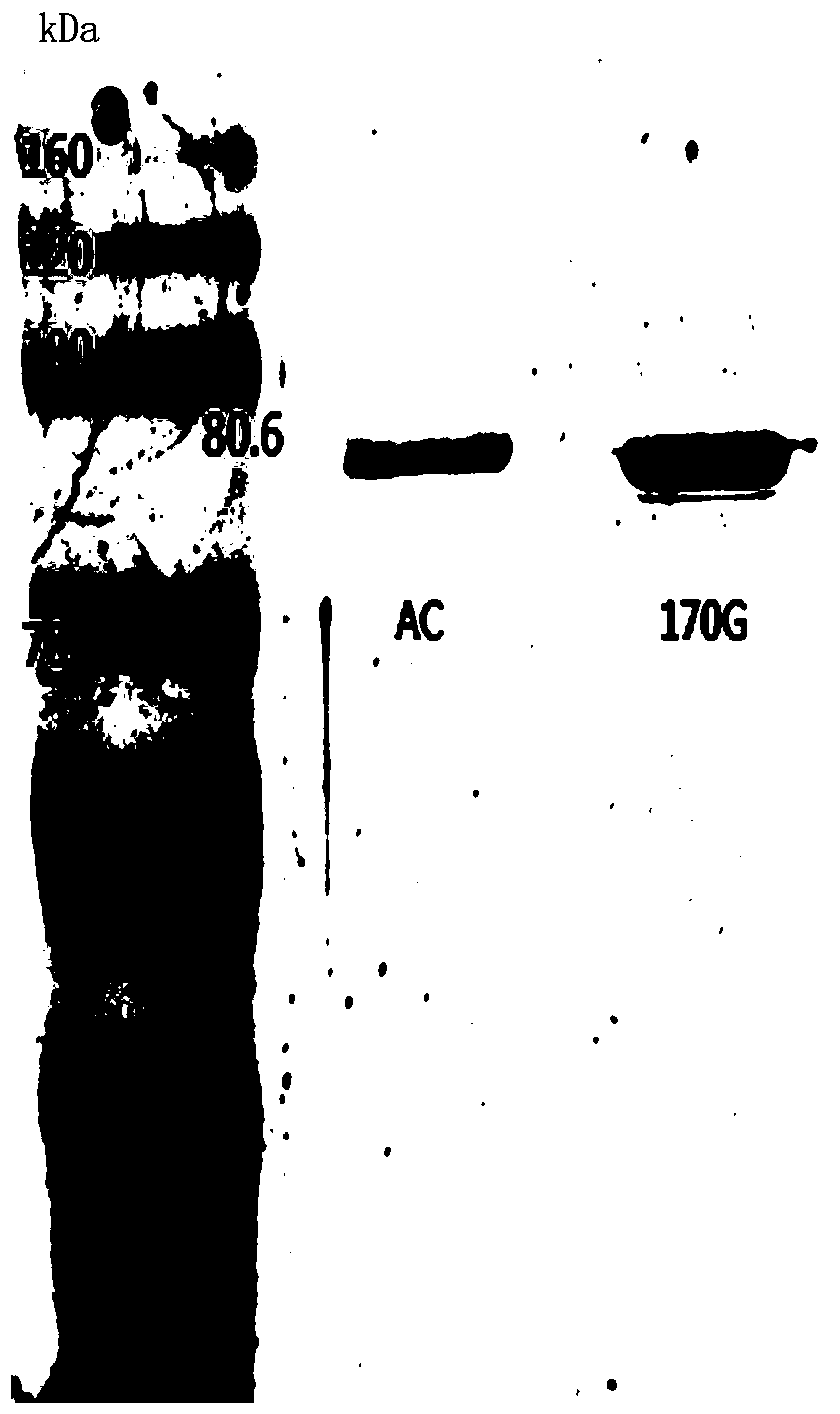
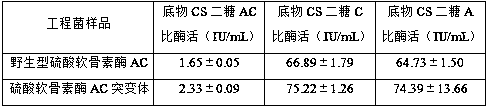
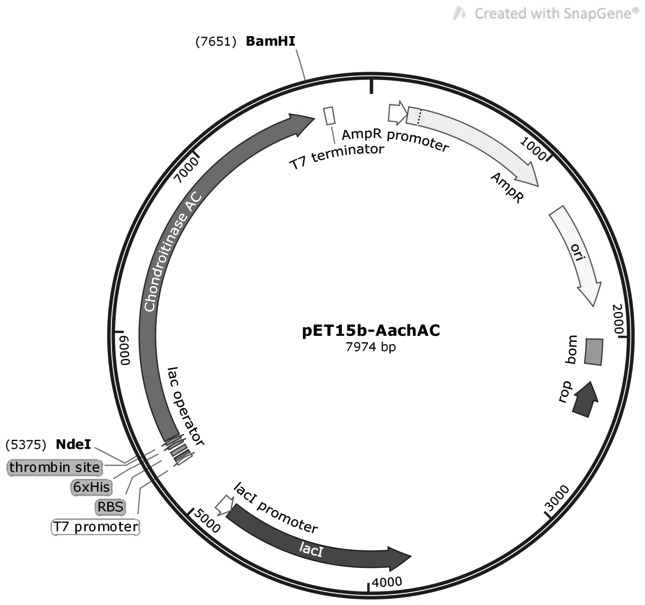
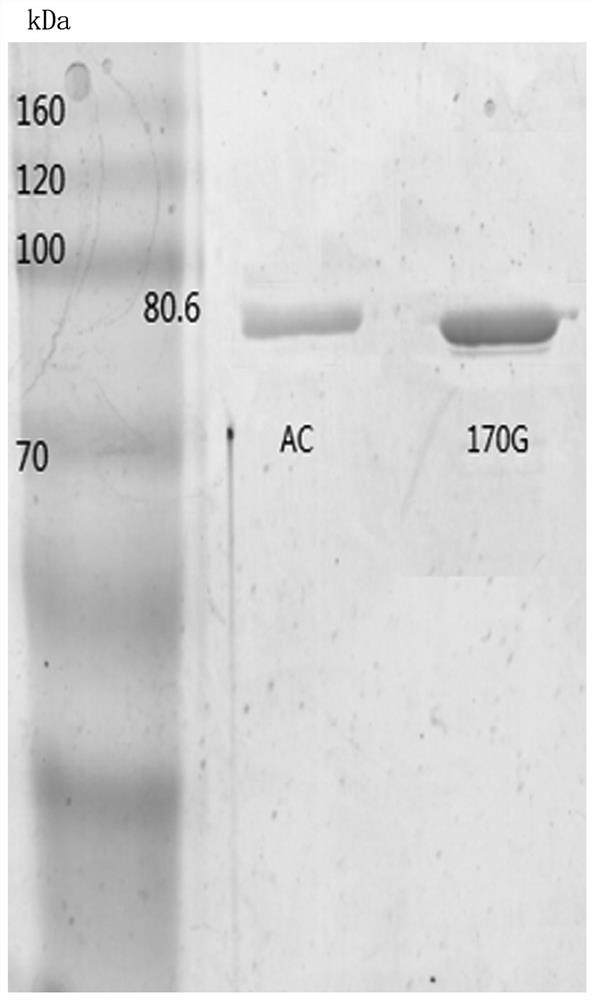
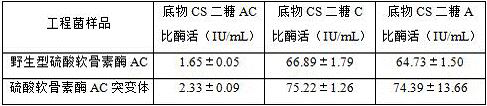
Chondroitinase AC mutant, encoding gene, vector, engineering bacterium and preparation method of chondroitinase AC mutant
ActiveCN110777140AHigh activityReduce binding steric hindranceBacteriaMicroorganism based processesGlycineNucleotide
The invention provides a chondroitinase AC mutant and a preparation method thereof. The mutant has an amino acid sequence as shown in SEQ ID NO: 2. The invention provides an encoding gene of the chondroitinase AC mutant. The encoding gene has a nucleotide sequence shown as shown in SEQ ID NO: 3. The invention further provides a recombinant vector and engineering bacterium of the chondroitinase ACmutant. Meanwhile, the invention also discloses a preparation method of the chondroitinase AC mutant. The 170th site in the amino acid sequence SEQ ID NO: 1 of wild chondroitinase AC 1 is mutated intoglycine to construct the chondrosulphatase AC mutant, so that the steric hindrance of the combination of the chondrosulphatase AC mutant and a polysaccharide substrate is reduced, the combination pocket of the chondrosulphatase AC mutant and polysaccharide is enlarged, the chondrosulphatase AC mutant can be more easily combined with the polysaccharide substrate, and the activity of chondrosulphatase AC is improved.
Owner:BEIJING POLYTECHNIC
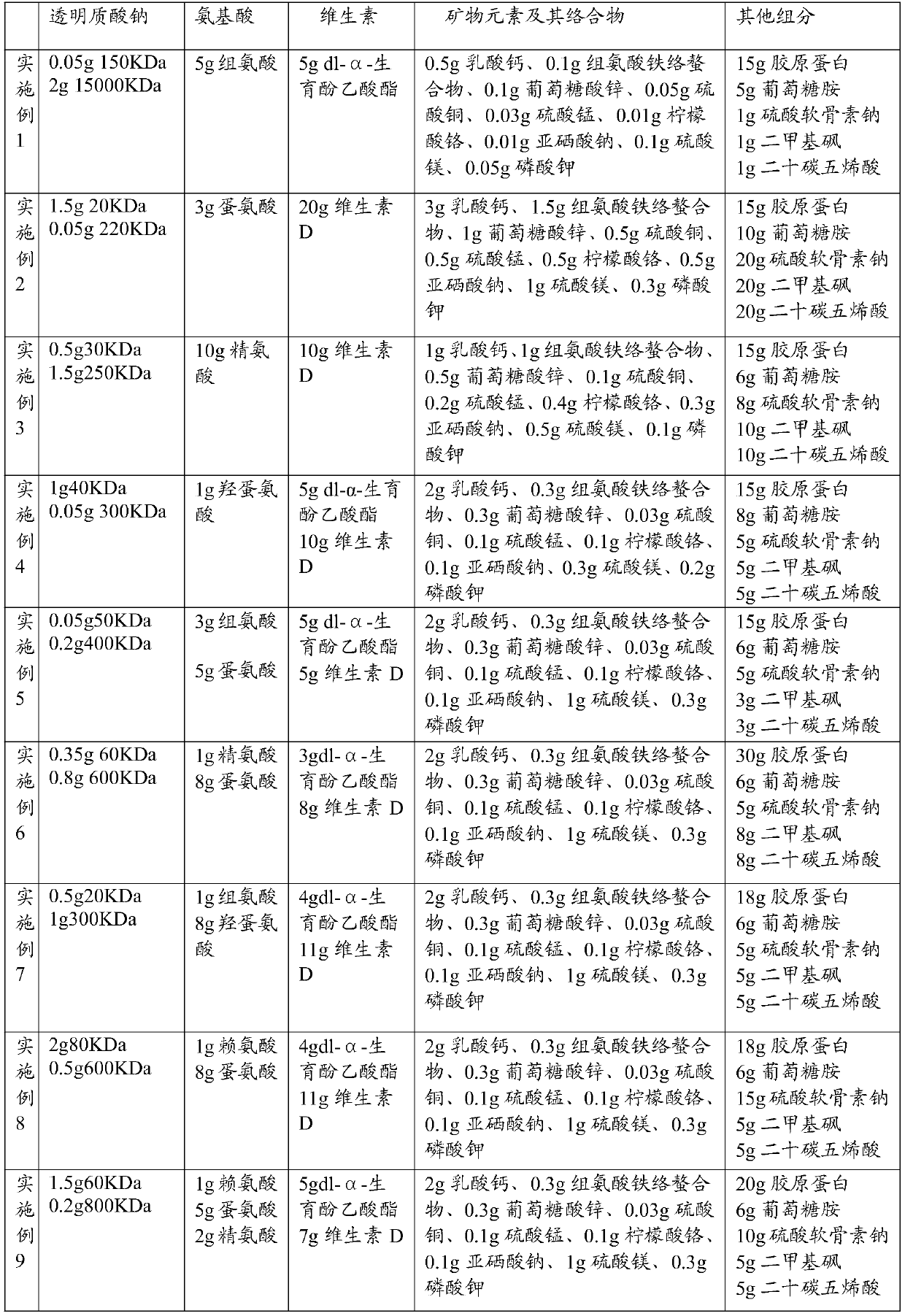
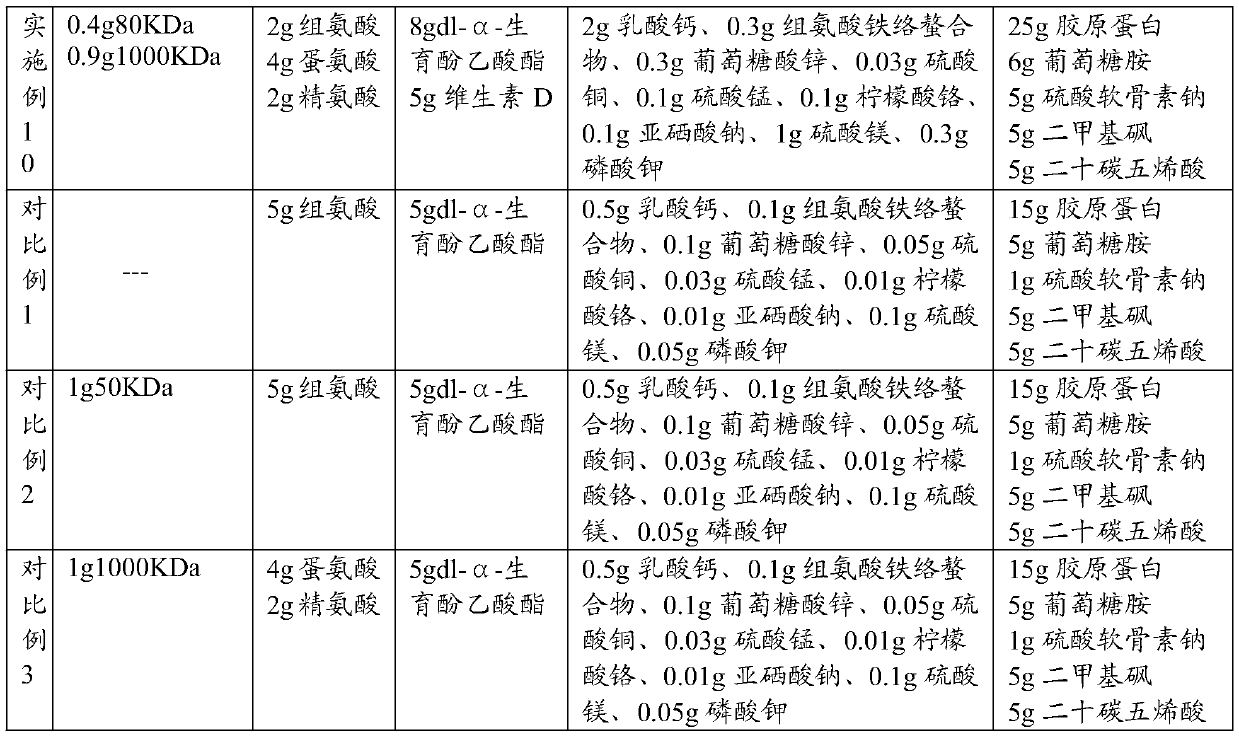
Composition containing sodium hyaluronate, preparation method of composition and application of composition
PendingCN109965104AImprove the lubrication effectGood prevention effectAccessory food factorsBone densityChondroitin sulphate
The invention discloses a composition containing sodium hyaluronate. The composition comprises first sodium hyaluronate with the molecular weight of 20KDa-150KDa, second sodium hyaluronate with the molecular weight of 220KDa-1500KDa, amino acid and vitamins. According to the composition containing the sodium hyaluronate, the first sodium hyaluronate and the second sodium hyaluronate are compoundedto serve as main components, and the sodium hyaluronate can effectively lubricate joints and is matched with the amino acid, the vitamins, mineral elements and complexes thereof, proteins, glucosamine, chondroitin sulphate sodium, dimethyl sulfone and timnodonic acid, so that the composition integrates calcium supplementation and joint protection, functions in effectively preventing arthritis andjoint movement damage, can promote development, enhance bone density and improve the toughness and the hardness of bones.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
Compounds having magnetic functionality, implants or gels derived from same, and use of both in order to determine the enzyme activity of an enzyme
InactiveCN104540529AMicrobiological testing/measurementDisease diagnosisCarboxyl radicalCell-Extracellular Matrix
The invention relates to an implant or gel including at least one compound having magnetic functionality, which in turn includes a substrate of at least one enzyme involved in regenerating the extracellular matrix and a plurality of magnetic particles. The invention also relates to a compound having magnetic functionality which includes a substrate of at least one enzyme involved in regenerating the extracellular matrix and a plurality of magnetic particles, preferably with no coating or surface modification, or functionalised with carboxyl groups, for the production of an implant or gel for monitoring an enzymatic activity involved in regenerating the extracellular matrix. The substrate is preferably selected from the group that comprises collagen, chondroitin sulphate and hyaluronic acid, and the magnetic particles are superparamagnetic particles.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC)
Chinese patent medicine for treating osteoarthritis and preparation method thereof
InactiveCN104383301AQuick cureEliminate odorOrganic active ingredientsAntipyreticCentipedeChondroitin sulphate
The invention belongs to the technical field of traditional Chinese medicines, relates to a Chinese patent medicine for treating osteoarthritis and a preparation method thereof. The Chinese patent medicine is prepared from the following raw materials in parts by weight: 5-10 parts of chondroitin sulphate sodium, 5-10 parts of glucosamine, 5-10 parts of burdock, 5-10 parts of salviae miltiorrhiza, 2-5 parts of eucommia ulmoides, 2-5 parts of earthworm, 2-5 parts of parasitic loranthus, 2-5 parts of radix achyranthis bidentatae, 2-5 parts of radix angelicae pubescentis, 2-5 parts of asarum, 2-5 root of largeleaf gentian, 2-5 parts of divaricate saposhnikovia root, 2-5 parts of ligusticum wallichii, 2-5 parts of dipsacus root, 2-5 parts of centipede, 2-5 parts of gastrodia elata and 2-5 parts of liquorice. The preparation method comprises the following steps: weighing raw materials in parts by weight; crushing into fine powder, sieving through an 80-100mesh sieve, and enclosing 0.6-0.7g of powder into each hollow capsule; and bottling and sealing. The traditional Chinese medicine components disclosed by the invention are capable of assisting the functions of chondroitin sulphate sodium and glucosamine for repairing cartilago articularis when developing the efficacy of strengthening muscles and bones; and the components of the traditional Chinese medicine and the Western medicine work together to quickly treat the osteoarthritis.
Owner:SHANDONG PROVINCIAL HOSPITAL
A method for extracting biologically active substances from eggshell membranes
InactiveCN104292364BImprove hydrolysis efficiencyNutritional value fully preservedPeptide preparation methodsFermentationTetrafluoroborateEggshell membrane
Owner:ZHEJIANG UNIV
Compositions Comprising Glycosaminoglycan and Nonsteroidal Anti-Inflammatory Drug
InactiveUS20080261866A1Minimizing gastric toxicityProvide effectBiocidePeptide/protein ingredientsGastric toxicityChondroitin sulphate
Pharmaceutical compositions comprising of glycosaminoglycan or salts thereof, preferably chondroitin or salts thereof, more preferably chondroitin sulphate, and nonsteroidal anti-inflammatory drug(s) or salts thereof, optionally with pharmaceutically acceptable excipient(s) are described. The compositions of the present invention provide gastrosparing effect in conditions where nonsteroidal anti-inflammatory drug(s) or their salts are used, particularly in mammals. Also provided are process for the manufacture of such novel compositions and method to minimize the nonsteroidal anti-inflammatory drug(s) induced gastric toxicity.
Owner:PANACEA BIOTEC
Combined porcine transmissible gastroenteritis and porcine epizootic diarrhea living vaccine cryoprotectant and combined living vaccine
ActiveCN110801436AFlat surfaceLittle price lossPowder deliverySsRNA viruses positive-senseSucroseChondroitin sulphate
The invention provides a combined porcine transmissible gastroenteritis and porcine epizootic diarrhea living vaccine cryoprotectant and a combined living vaccine. The cryoprotectant is prepared fromthe following components in percentage by mass volume: 20-28 percent of cane sugar, 6-10 percent of gelatin, 2-6 percent of tryptone, 1-3 percent of yeast extract powder, 0.1-2 percent of chondroitinsulfate, 1-5 percent of inositol and the balance being injection water, based on the unit of mass volume ratio being g / ml. The combined living vaccine comprises the cryoprotectant, the cryoprotectantcan effectively solve the problem that the surface of an existing lyophilized product has pits and cracks, and the lyophilized product has low dissolving speed and titer loss after lyophilization.
Owner:SICHUAN HUASHEN ANIMAL BIOLOGICAL PRODS
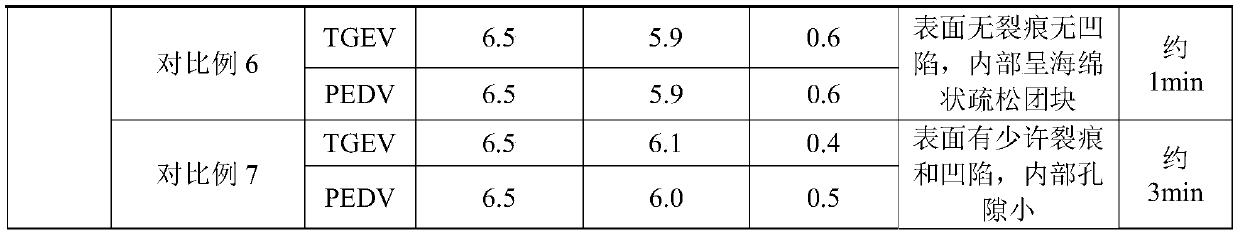
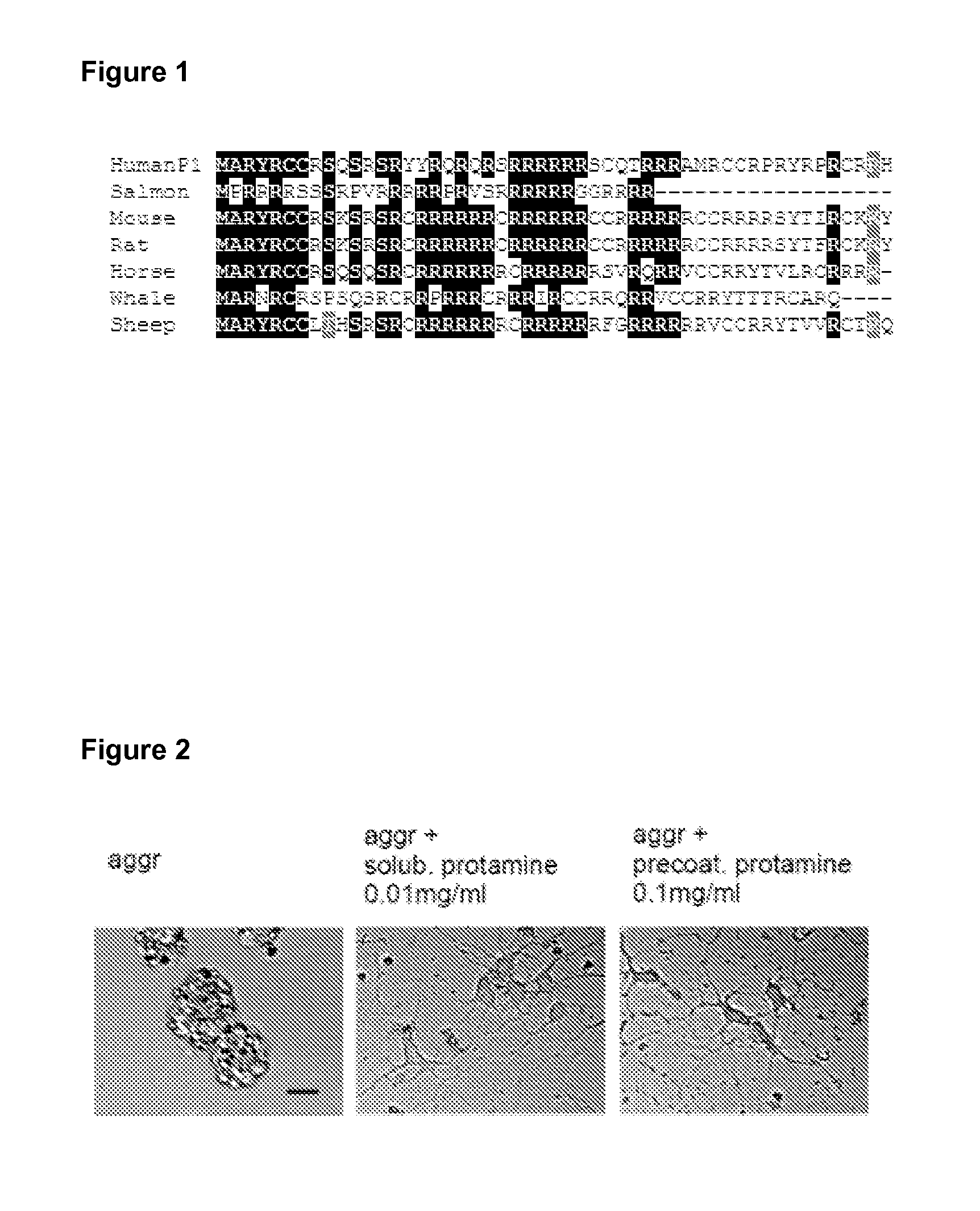
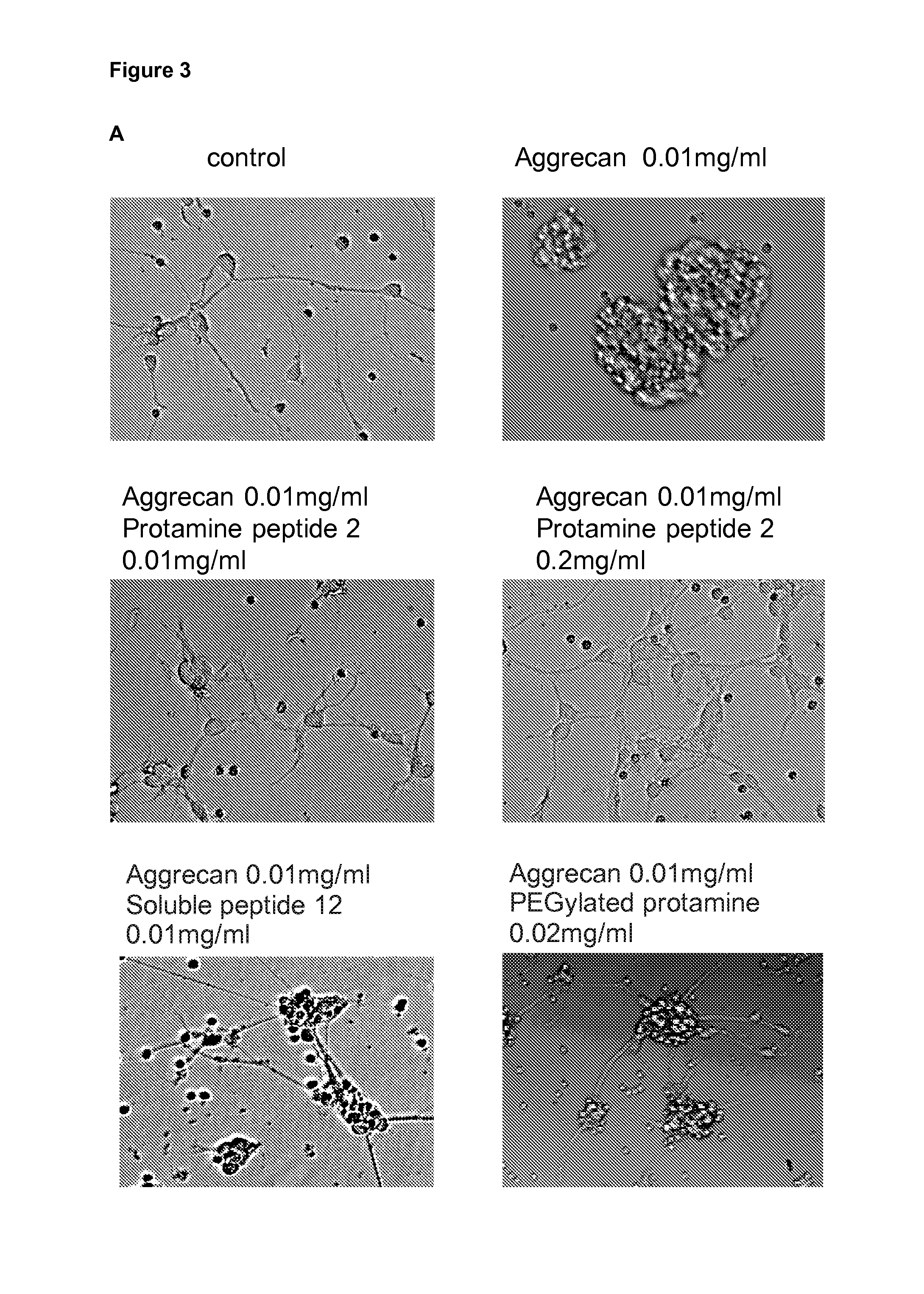
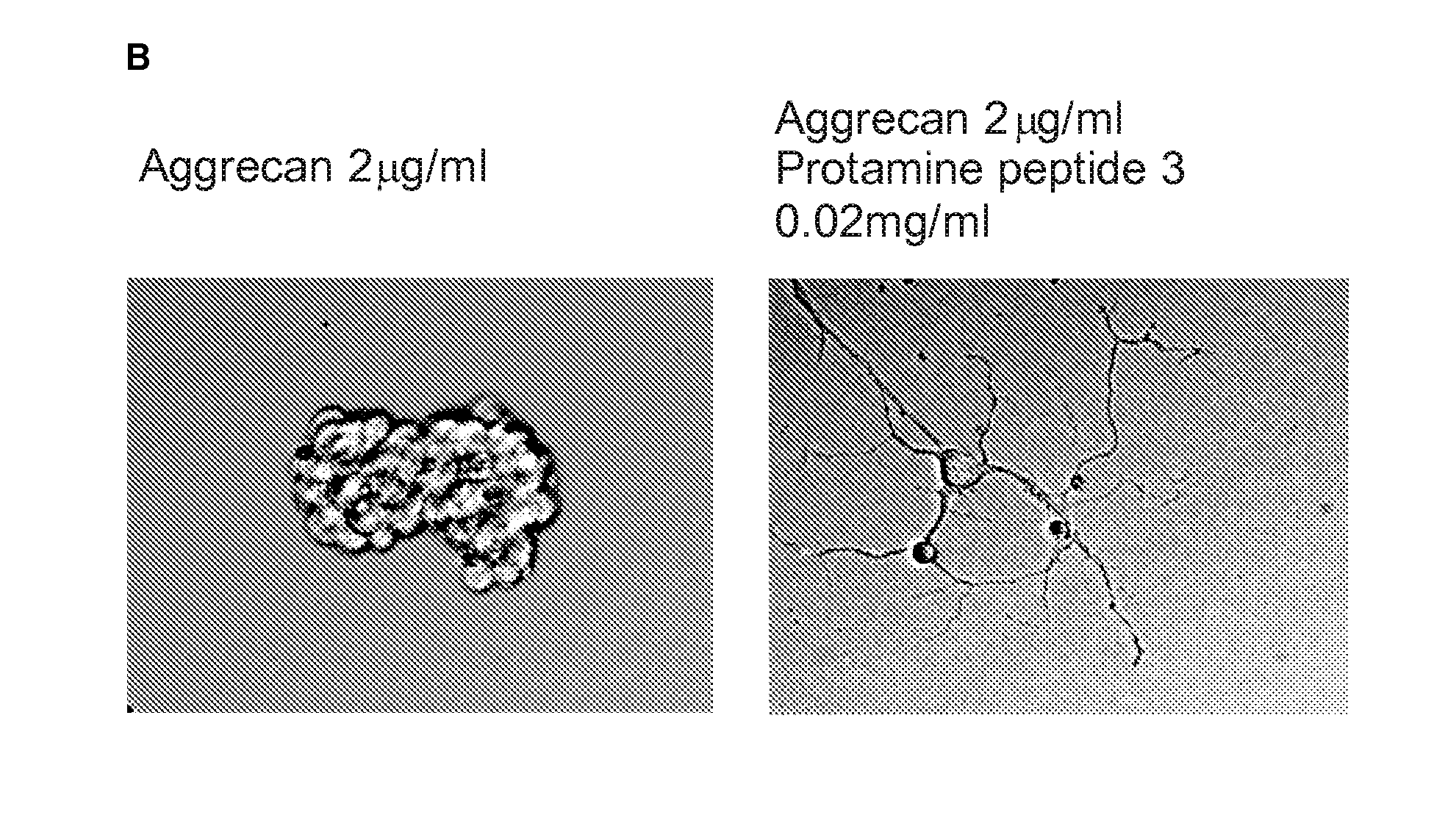
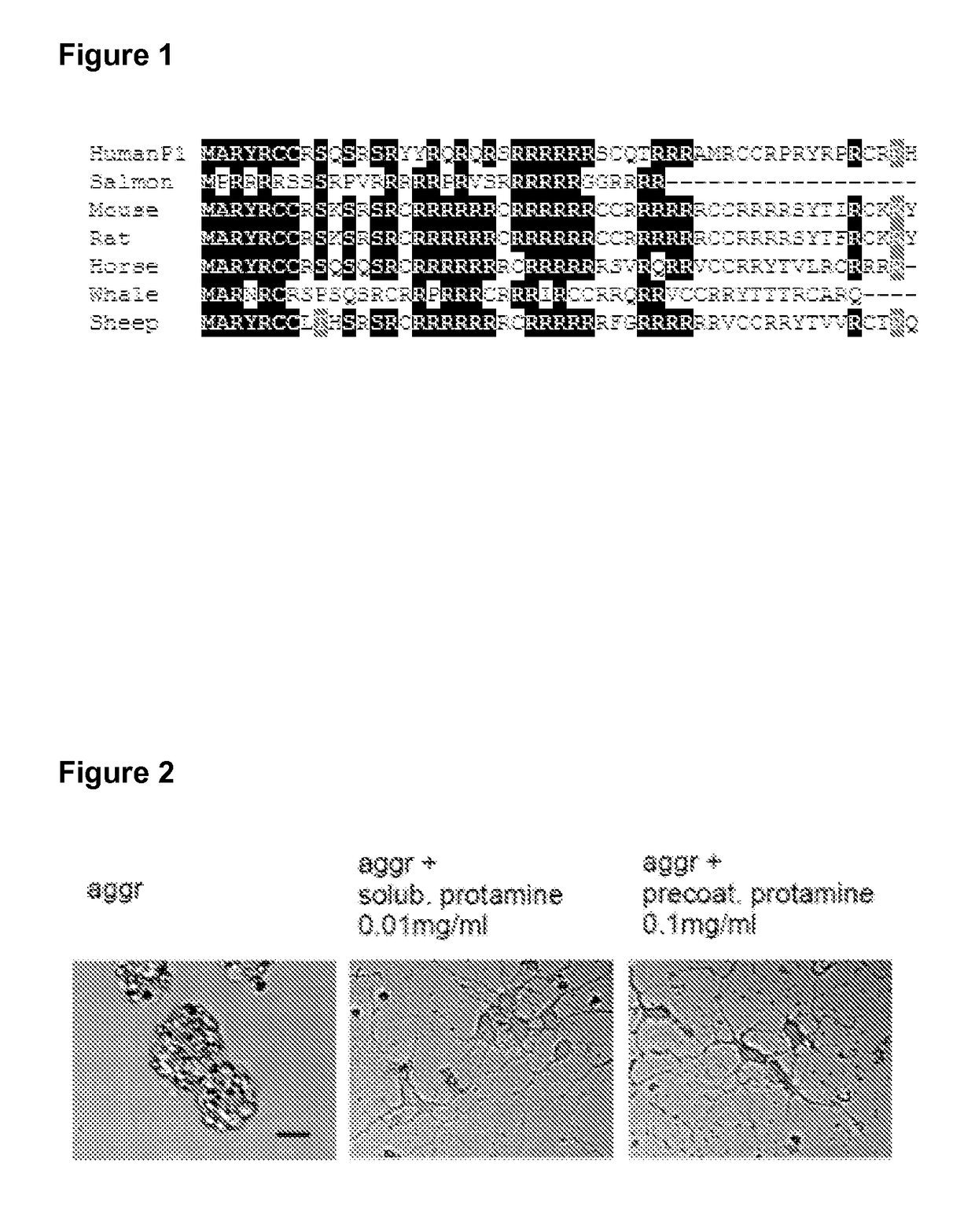
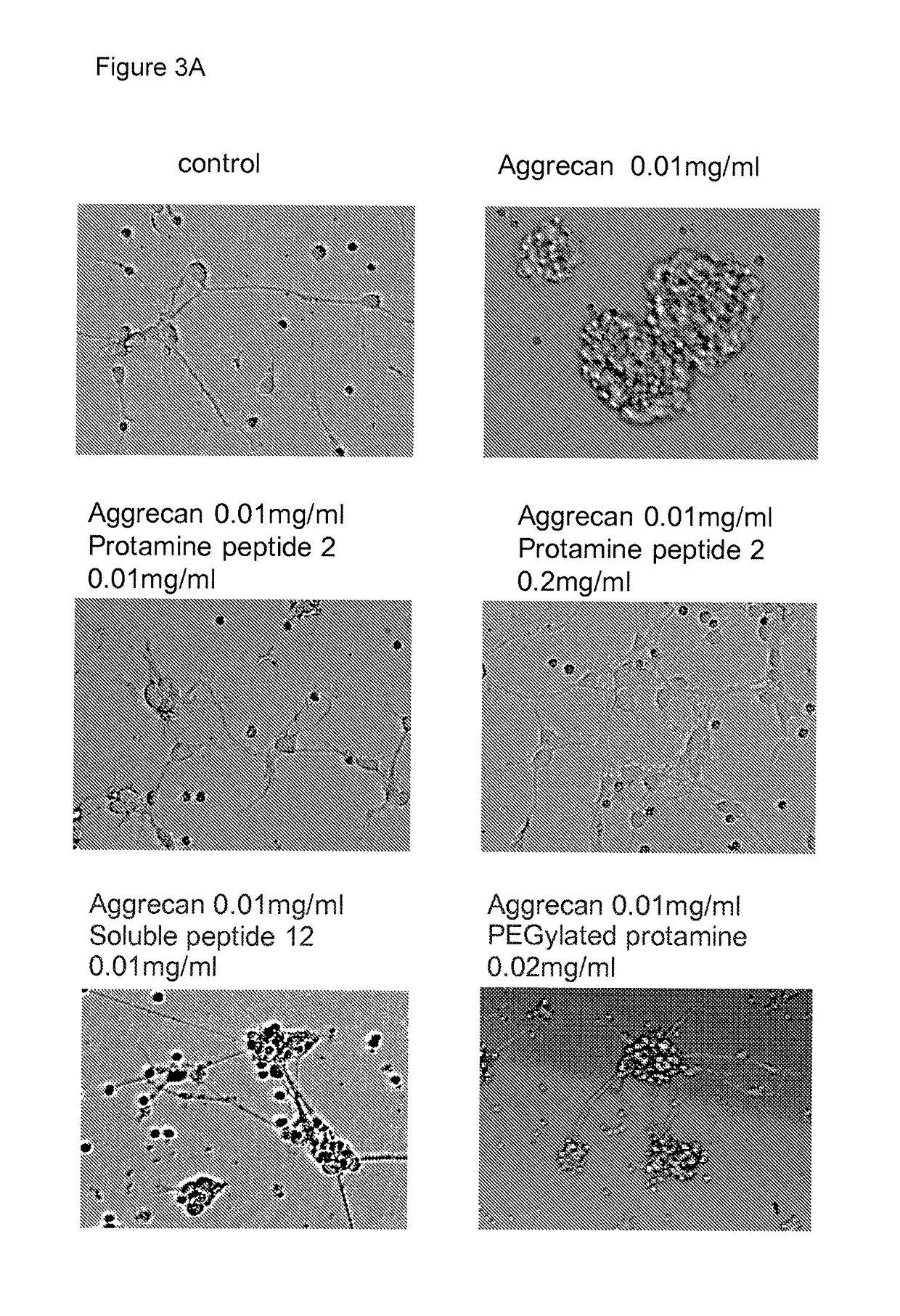
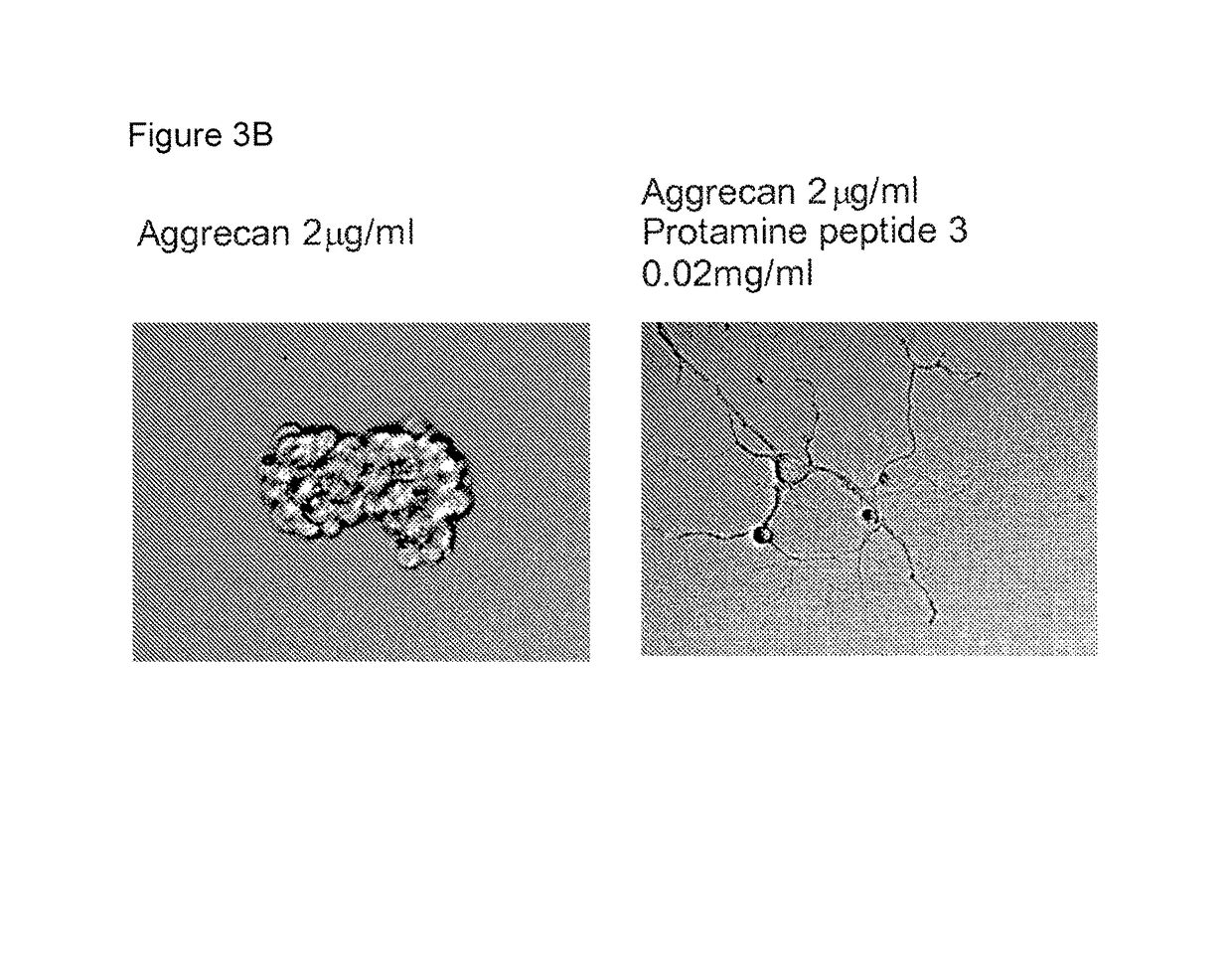
Protamine in treatment of neuronal injuries
ActiveUS20160272689A1Promotes CNS regenerationPromote regenerationNervous disorderPeptide/protein ingredientsNeuronal damageChondroitin sulphate
The present invention relates to treatment of neuronal injury. The present invention discloses a novel use of an agent and a novel method for promoting neurite out growth and / or neural regeneration in CNS injuries. A novel mechanism of promoting neurite outgrowth by increasing the interaction of chondroitin sulphate proteoglycan (CSPG) to receptor protein tyrosine phosphatase sigma (RPTPσ) is disclosed.
Owner:UNIVERSITY OF HELSINKI
Oyster mushroom soy sauce and preparation method thereof
InactiveCN106616855AIncreased ethyl acetate contentNatural extract food ingredientsFood ingredient functionsSalvia miltiorrhizaRadix Astragali seu Hedysari
The invention provides an oyster mushroom soy sauce. The oyster mushroom soy sauce is characterized by being prepared from the following raw materials and additives in parts by weight: 200 to 300 parts of soy sauce, 18 to 30 parts of oyster mushroom, 20 to 40 parts of radix astragali seu hedysari, 18 to 25 parts of salvia miltiorrhiza, 10 to 20 parts of dark plum, 12 to 20 parts of liquorice root, and 6 to 15 parts of additive, wherein the additive comprises the following components in parts by weight: 1 to 4 parts of 2-ethyl-3,5-dimethylpyrazine, 2 to 4 parts of sodium alginate, and 3 to 7 parts of chondroitin sulphate sodium. The oyster mushroom soy sauce has the advantage that after placing, the contents of amino acid and acetic ether are higher.
Owner:南京康凯生物科技有限公司
Composition for repairing epithelial injury of bladders and urinary tracts
InactiveCN104940221AExcellent physical and chemical propertiesHigh biosecurityOrganic active ingredientsAntipyreticUrethraChondroitin sulphate
The invention relates to a composition for repairing epithelial injury of bladders and urinary tracts. The composition is composed of two or three components selected from a group including hyaluronic acid, chitosan and chondroitin sulphate. Clinical animal experiments show that the composition for repairing epithelial injury of bladders and urinary tracts disclosed by the invention is capable of obviously repairing epithelial injury of bladders due to protamine sulphate or cyclophosphamide, obviously reducing the bladder epithelial injury range and the injury degree and lightening the inflammatory infiltration degree.
Owner:BEIJING DATSING BIO TECH
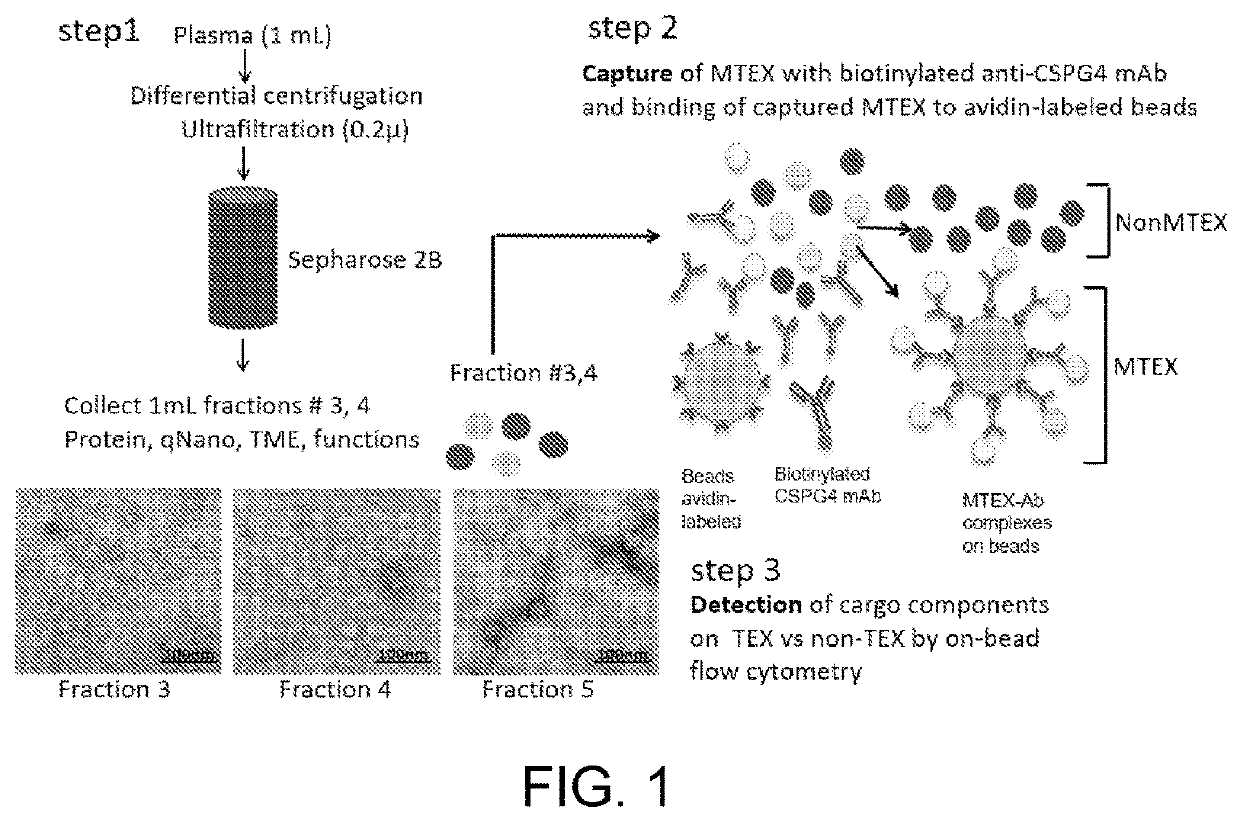
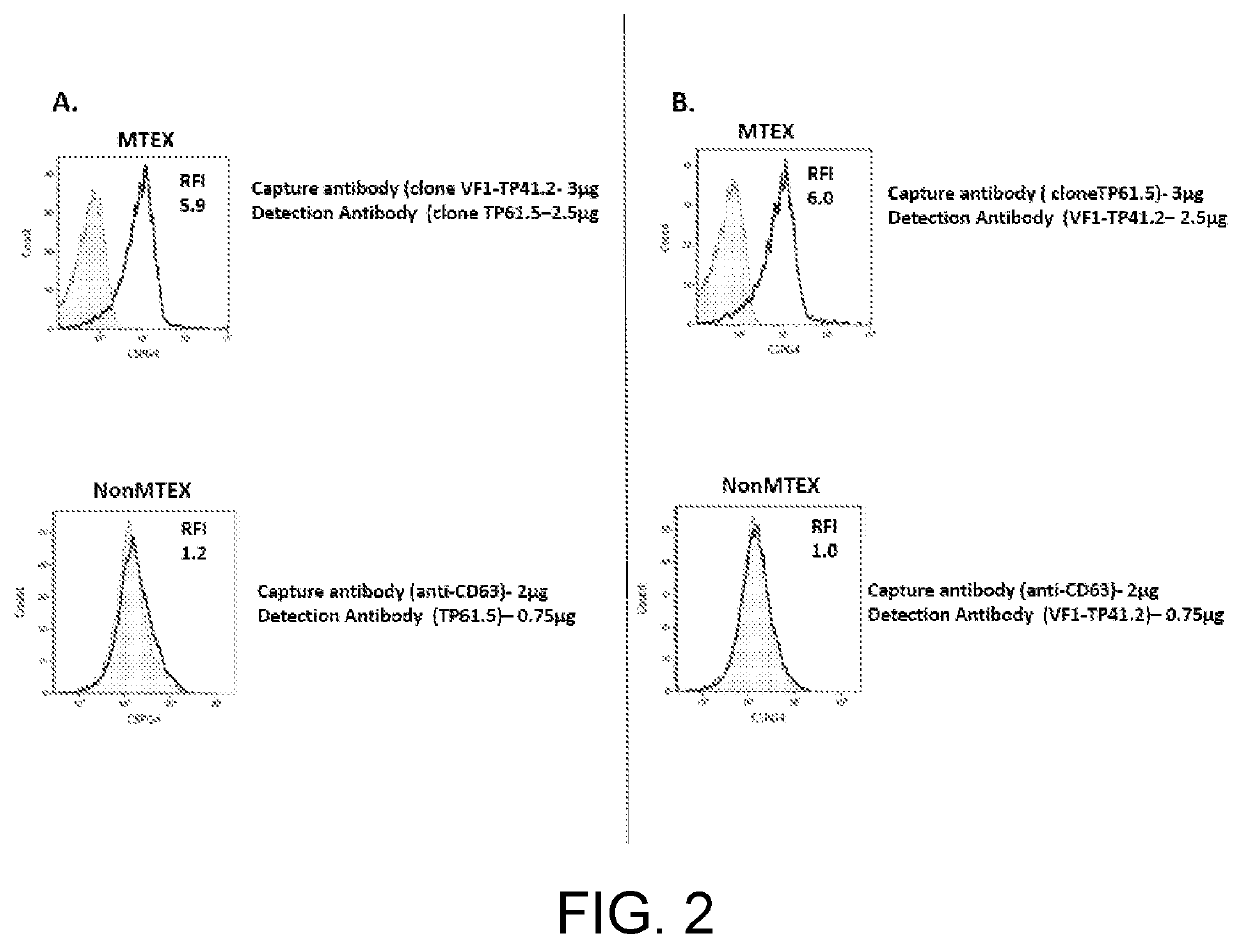
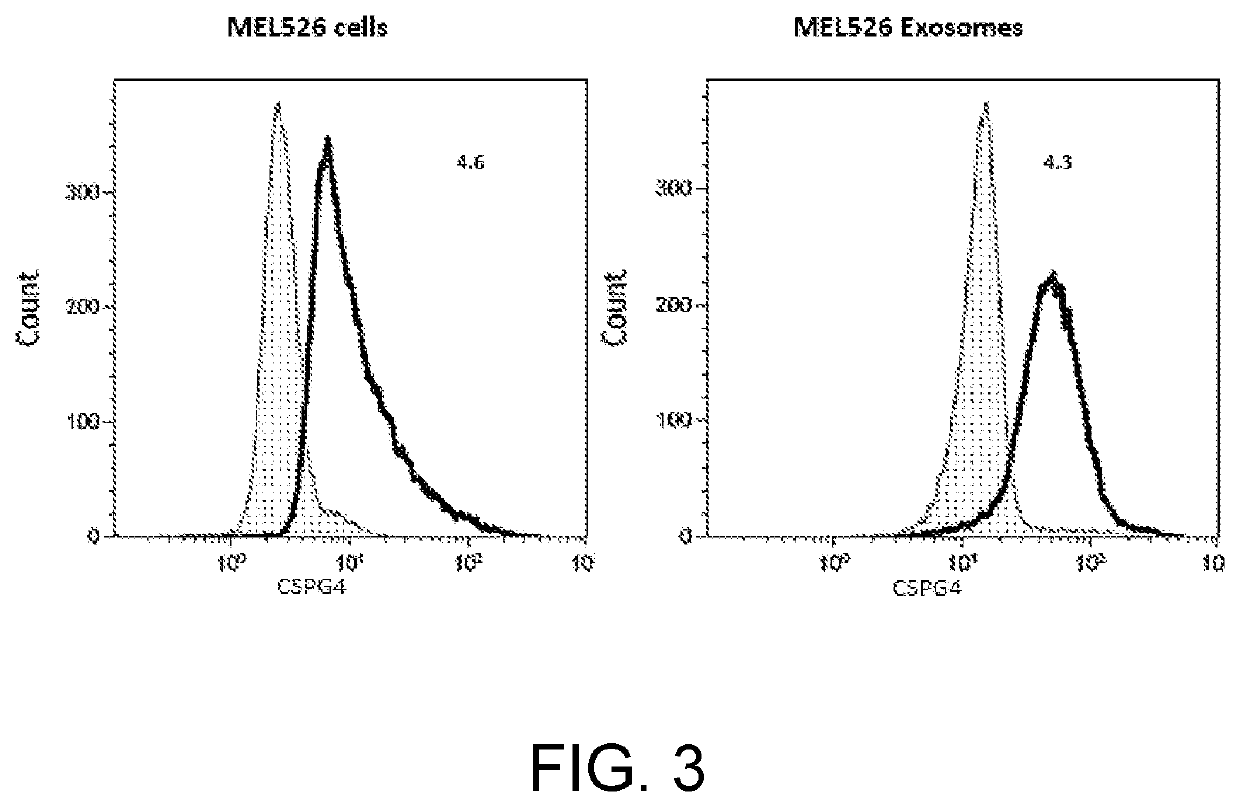
Tumor cell-derived exosomes and their applications
PendingUS20220229059A1Assess prognosisMonitor responseComponent separationImmunoglobulinsCSPG4Antigen
The disclosure features compositions and methods that may be used to detect the presence of tumor cell-derived exosomes in a patient (e.g., a human patient) having cancer. The compositions and methods described herein may also be used to evaluate the patient's prognosis, as well as monitor the likelihood of the patient to benefit from therapy, such as immunotherapy. The disclosure also features antibodies that specifically bind chondroitin sulphate proteoglycan 4 (CSPG4), as well as antigen-antibody complexes containing the same.
Owner:THE GENERAL HOSPITAL CORP +1
Chondroitinase ac mutant and preparation method thereof
ActiveCN110777140BHigh activityReduce binding steric hindranceBacteriaMicroorganism based processesGlycineChondroitin sulphate
The present invention provides a chondroitinase AC mutant, the amino acid sequence of which is shown in SEQ ID NO:2. The present invention also provides a method for preparing the AC mutant of chondroitinase sulfate. The present invention constructs the chondroitinase AC mutant by mutating the 170th position in the wild-type chondroitinase AC amino acid sequence SEQ ID NO:1 to glycine, thus reducing the chondroitinase AC mutant and polysaccharide The steric hindrance of substrate binding expands its binding pocket with polysaccharides, making it easier to bind polysaccharide substrates, thereby increasing the activity of chondroitinase AC.
Owner:BEIJING POLYTECHNIC
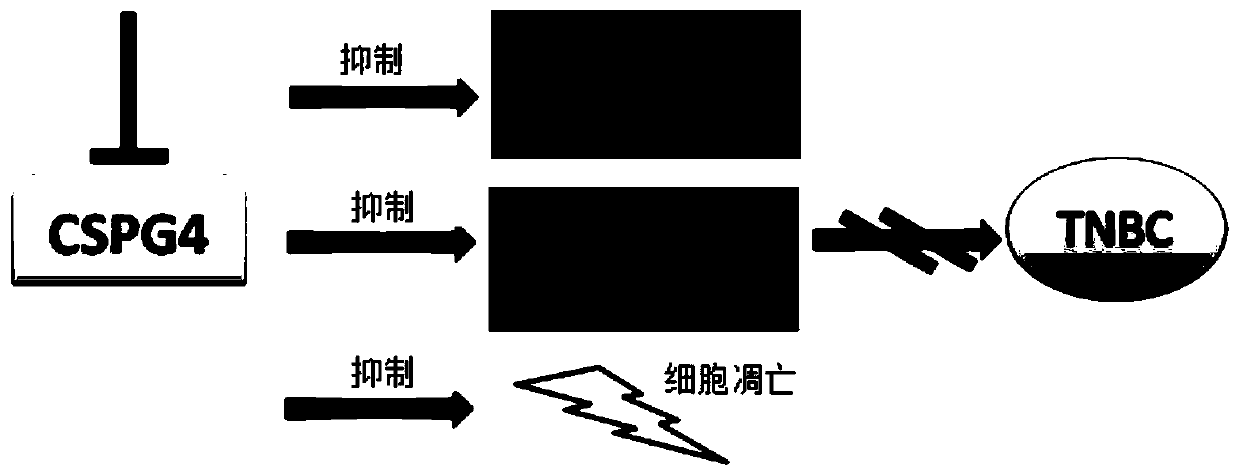
Application for using CSPG4 (Chondroitin Sulphate Proteoglycan 4) as molecular target in treatment of triple-negative breast cancer
PendingCN110389215AEffective treatmentImmunoglobulins against animals/humansAntibody ingredientsCSPG4Chondroitin sulphate
The invention researches a treatment mechanism for using the CSPG4 (Chondroitin Sulphate Proteoglycan 4) as the molecular target of the immunotherapy in the treatment of the triple-negative breast cancer through the theoretical research and the experiments in vivo and vitro, provides an effective treatment of the triple-negative breast cancer, and simultaneously provides a theoretical basis for the application for using the CSPG4 (Chondroitin Sulphate Proteoglycan 4) as the antibody in the treatment of the triple-negative breast cancer.
Owner:阳剑波
Composition for the treatment of osteoarthritis
ActiveUS20100286086A1Increase capacityIncrease elasticityBiocideOrganic active ingredientsDiseaseChondroitin sulphate
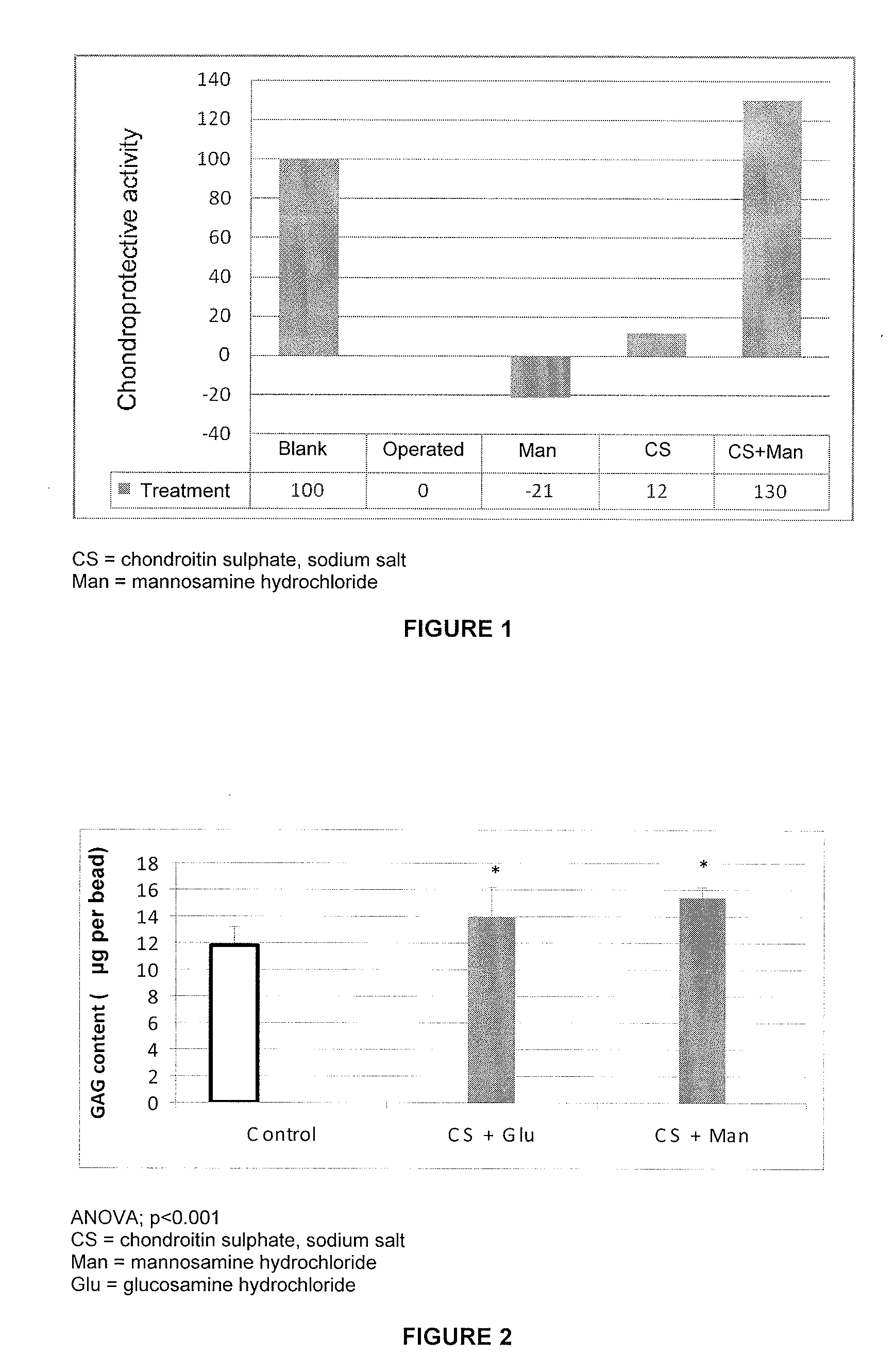
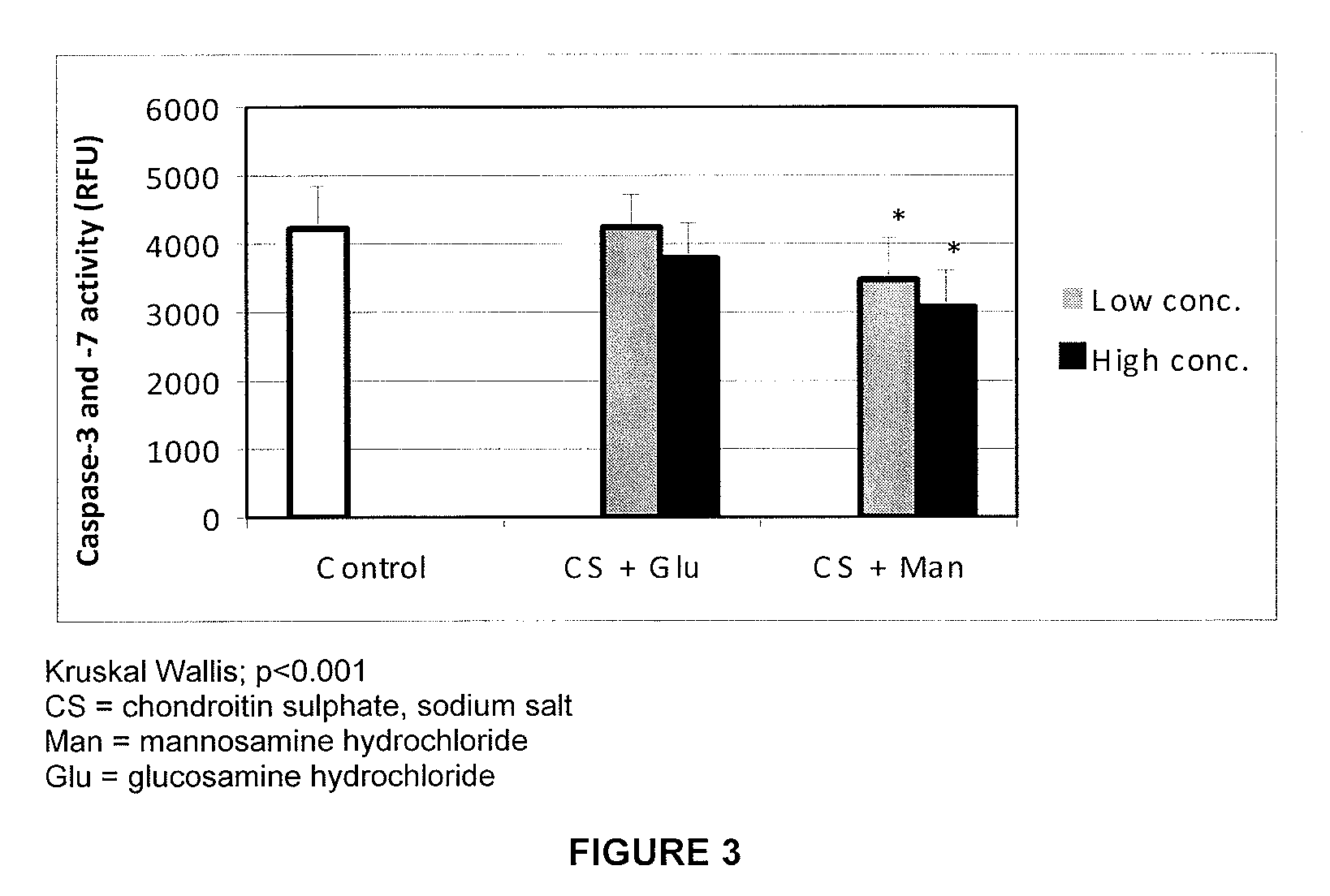
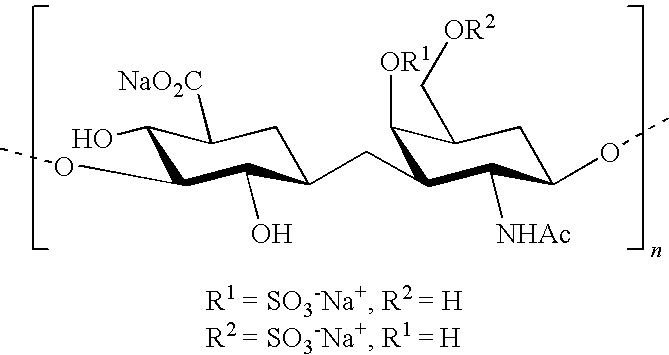
The present invention relates to compositions comprising chondroitin sulphate and mannosamine or a derivative thereof. The mannosamine derivative is preferably N-acetylmannosamine. The compositions may comprise glucosamine. Said compositions are useful in the treatment or prevention of degenerative joint diseases, preferably of osteoarthritis, in the treatment or prevention of tendon or ligament diseases, disorders or injuries and of immune system diseases, preferably of rheumatoid arthritis.
Owner:BIOIBI12RICA
Protamine in treatment of neuronal injuries
ActiveUS9896488B2Promote regenerationSafety/toxicity concerns have been addressedNervous disorderPeptide/protein ingredientsNeurophysinsNeuronal damage
The present invention relates to treatment of neuronal injury. The present invention discloses a novel use of an agent and a novel method for promoting neurite out growth and / or neural regeneration in CNS injuries. A novel mechanism of promoting neurite outgrowth by increasing the interaction of chondroitin sulphate proteoglycan (CSPG) to receptor protein tyrosine phosphatase sigma (RPTPσ) is disclosed.
Owner:UNIVERSITY OF HELSINKI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com