Corneal metaphase preserving liquid containing recombinant human serum albumin and preparation method thereof
A technology of human serum albumin and preservation solution, applied in the field of corneal mid-term preservation solution containing recombinant human serum albumin and its preparation, can solve problems such as exogenous pollution, improve the success rate, maintain collagen fiber structure and transparency , the effect of saving a long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
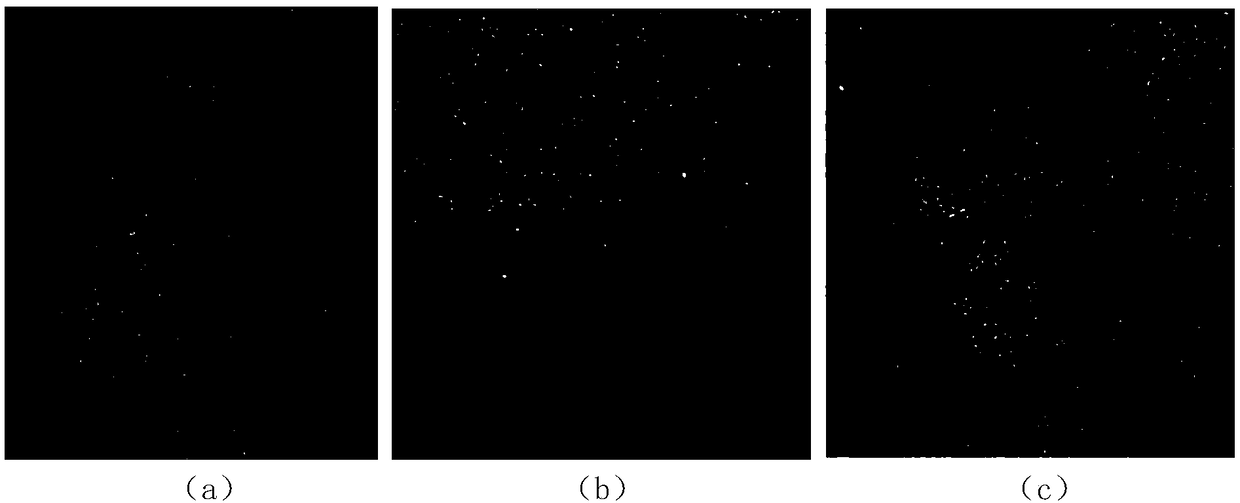
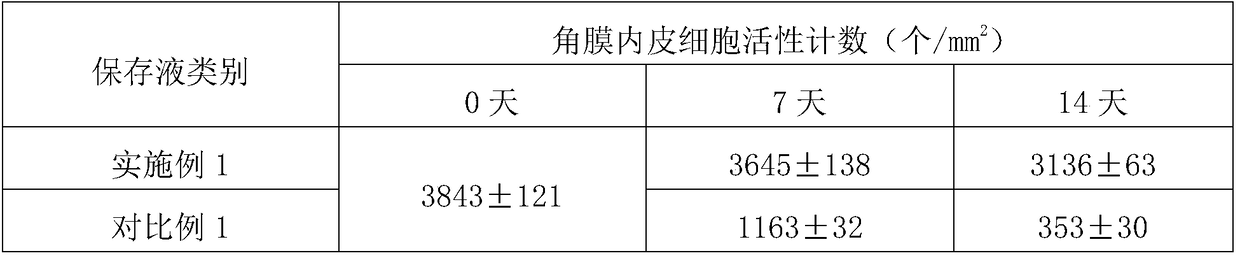
[0031] A medium-term corneal preservation solution containing recombinant human serum albumin, containing glutamine, calcium pantothenate, choline chloride, folic acid, inositol, nicotinamide, pyridoxal·HCl, riboflavin, thiamine·HCl , KCl, NaCl, NaH 2 PO 4 2H 2 O, glucose, dextran 40, sodium pyruvate, vitamin C, sodium chondroitin sulfate, sodium hyaluronate, non-animal source recombinant human serum albumin, gentamicin sulfate, sodium bicarbonate, 4-hydroxyethylpiperazine Ethylsulfonic acid, phenol red indicator, water for injection, arginine·HCl, cystine, histidine·HCl·H 2 O, isoleucine, leucine, lysine·HCl, methionine, phenylalanine, threonine, tryptophan, tyrosine, valine, of which water for injection is the solvent, other groups The concentrations of the points are: glutamine 0.6g / L, calcium pantothenate 0.002g / L, choline chloride 0.002g / L, folic acid 0.002g / L, inositol 0.004g / L, nicotinamide 0.001g / L, Pyridoxal·HCl 0.002g / L, riboflavin 0.0002g / L, thiamine·HCl 0.002g / ...
Embodiment 2
[0043] A medium-term corneal preservation solution containing recombinant human serum albumin, containing glutamine, calcium pantothenate, choline chloride, folic acid, inositol, nicotinamide, pyridoxal·HCl, riboflavin, thiamine·HCl , KCl, NaCl, NaH 2 PO 4 2H 2 O, glucose, dextran 40, sodium pyruvate, vitamin C, sodium chondroitin sulfate, sodium hyaluronate, non-animal source recombinant human serum albumin, gentamicin sulfate, sodium bicarbonate, 4-hydroxyethylpiperazine Ethylsulfonic acid, phenol red indicator, water for injection, arginine·HCl, cystine, histidine·HCl·H 2 O, isoleucine, leucine, lysine·HCl, methionine, phenylalanine, threonine, tryptophan, tyrosine, valine, of which water for injection is the solvent, other groups The concentrations of the points are: glutamine 0.3g / L, calcium pantothenate 0.002g / L, choline chloride 0.002g / L, folic acid 0.002g / L, inositol 0.004g / L, nicotinamide 0.001g / L, Pyridoxal·HCl 0.002g / L, riboflavin 0.0002g / L, thiamine·HCl 0.002g / ...
Embodiment 3
[0056] A medium-term corneal preservation solution containing recombinant human serum albumin, containing glutamine, calcium pantothenate, choline chloride, folic acid, inositol, nicotinamide, pyridoxal·HCl, riboflavin, thiamine·HCl , KCl, NaCl, NaH 2 PO 4 2H 2 O, glucose, dextran 40, sodium pyruvate, vitamin C, sodium chondroitin sulfate, sodium hyaluronate, non-animal source recombinant human serum albumin, gentamicin sulfate, sodium bicarbonate, 4-hydroxyethylpiperazine Ethylsulfonic acid, phenol red indicator, water for injection, arginine·HCl, cystine, histidine·HCl·H 2O, isoleucine, leucine, lysine·HCl, methionine, phenylalanine, threonine, tryptophan, tyrosine, valine, of which water for injection is the solvent, other groups The concentrations of the points are: glutamine 0.6g / L, calcium pantothenate 0.004g / L, choline chloride 0.004g / L, folic acid 0.004g / L, inositol 0.008g / L, nicotinamide 0.002g / L, Pyridoxal·HCl 0.004g / L, riboflavin 0.0004g / L, thiamine·HCl 0.004g / L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com