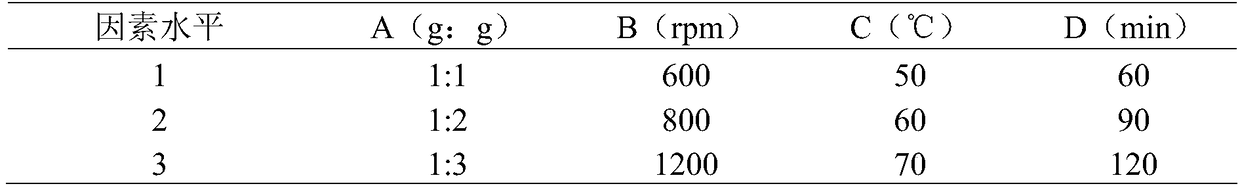
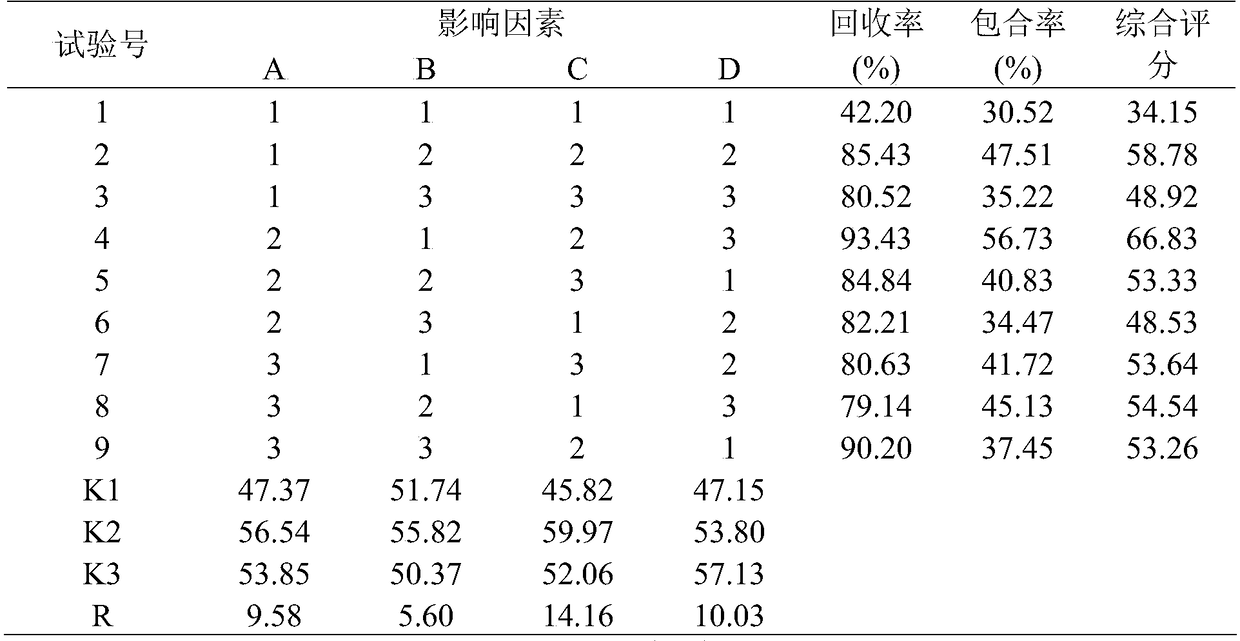
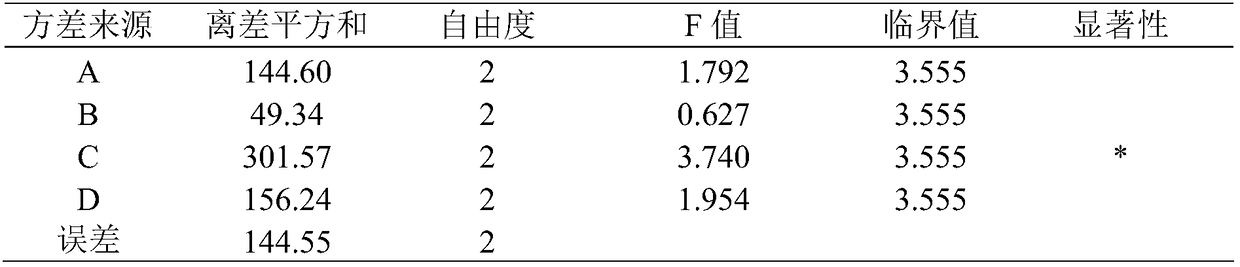
Patents
Literature
52 results about "Gentamicin Sulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
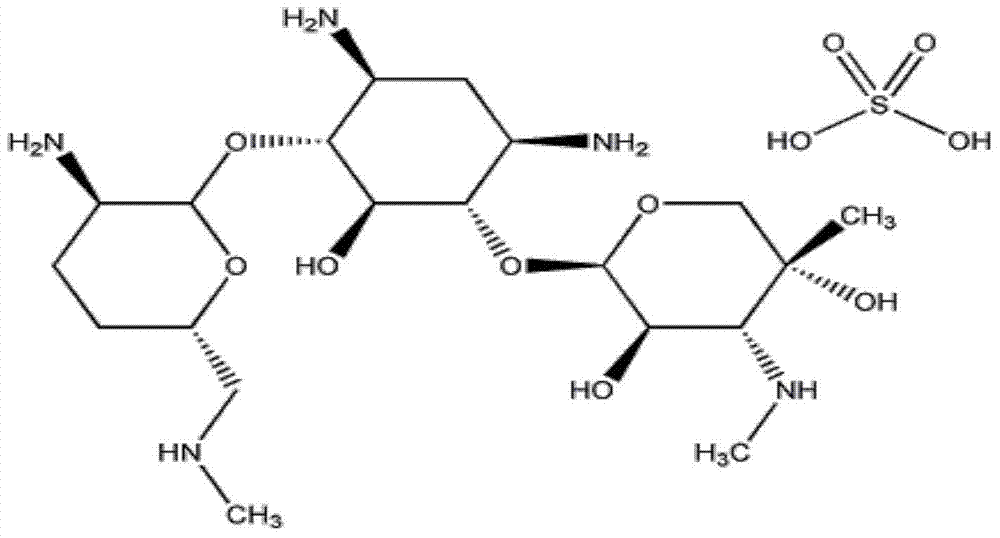
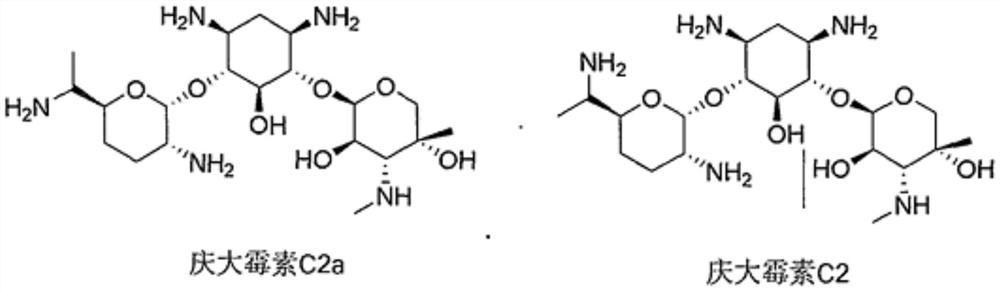
The sulfate salt form of gentamycin, a broad-spectrum aminoglycoside antibiotic complex produced by the fermentation of Micromonospora purpurea or M. echinospora, with antibacterial activity. Gentamicin is a thermostable complex containing the gentamicins C1, C1a, C2, C2a and C2b.
Hepatitis c virus antigen-antibody joint detection reagent box and preparation method thereof
ActiveCN104237520AThe "window period" is shortenedHigh detection specificityMaterial analysisAntigenPolyethylene glycol
A hepatitis c virus antigen-antibody joint detection reagent box is characterized by comprising a calibrator(1), a double-marker enzyme conjugate (2), a negative and positive contrast (3), light-emitting liquid (4) and a micropore coated plate (5), wherein the light-emitting liquid contains light-emitting liquid 1 and light-emitting liquid 2, the light-emitting liquid 1 contains luminal 0.7 g / L, cinnamic acid 0.9 g / L, 4-iodophenylboronic acid 0.2 g / L, iodobiphenol 0.25 g / L, dimethylformamide 25 ml / L, polyving akohol 5 g / L, polyvinylpyrrolidone 8 g / L, polyethylene glycol 600 3 g / L, ethylenediamine tetraacetic acid 4 g / L, gentamicin sulfate 1600 thousands / L, urea peroxide 0.4 g / L, and pH 9.0 Tris buffer solution 0.1 mol / L. The light-emitting liquid 2 contains acridinium ester derivative 0.1 mg / ml, polyethylene glycol 600 3 g / L and 0.1 mol / L of pH 9.0 Tris buffer solution containing 0.1% of TWEEN-20. The invention further discloses a preparation method and a using method of the reagent box. The hepatitis c virus antigen-antibody joint detection reagent box has the advantages of being quick in reaction and low in cost.
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
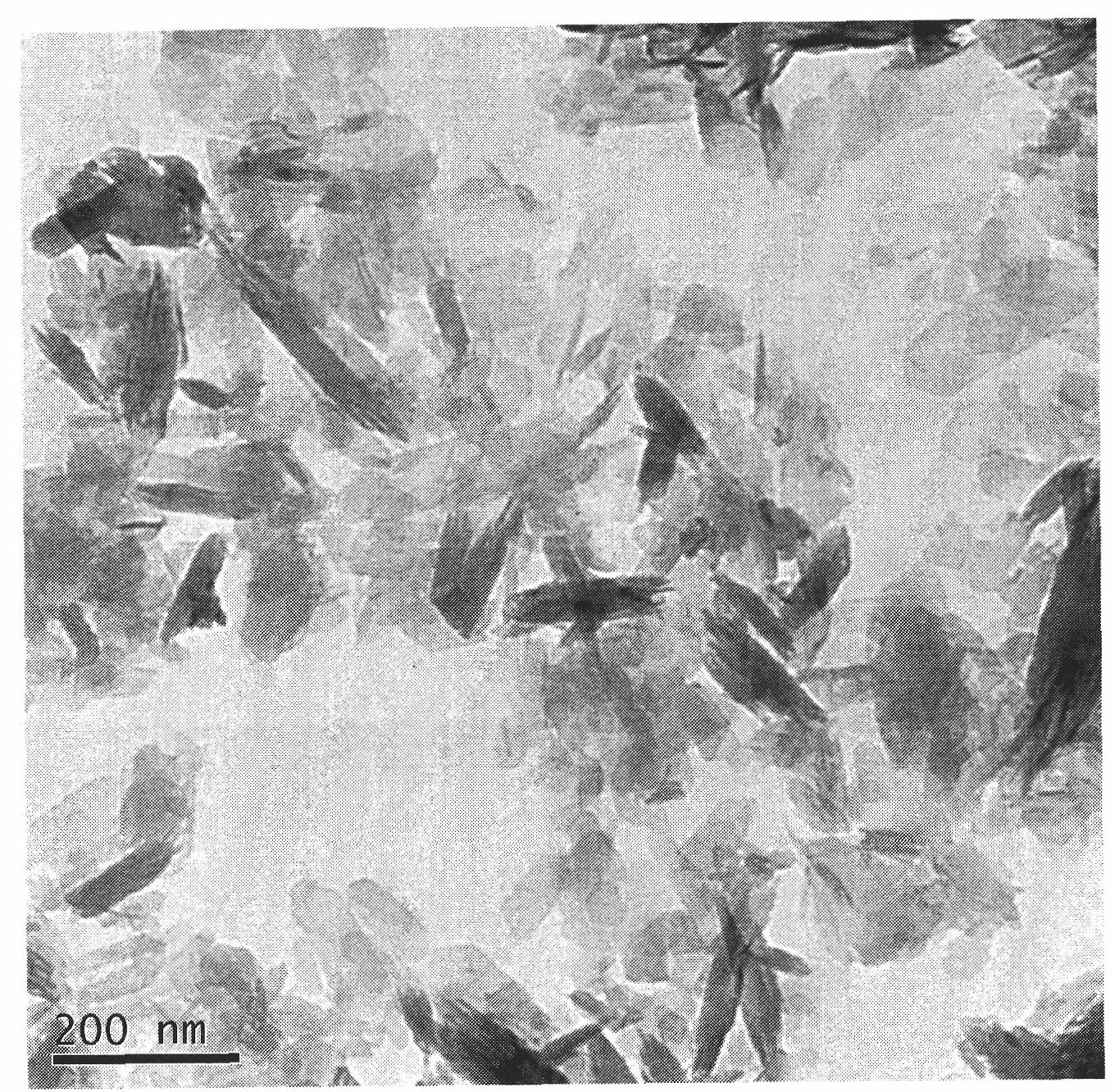
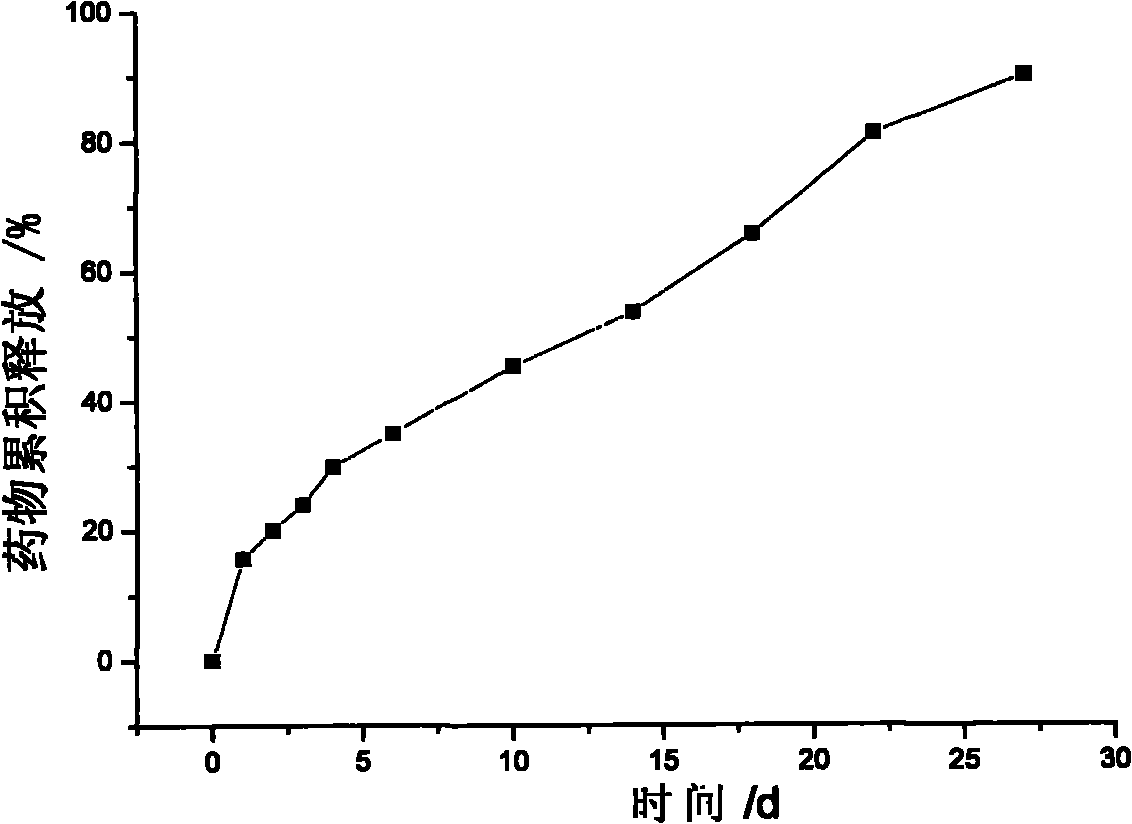
Gentamicin sulfate/gelatin microsphere complex-loaded silk fibroin scaffold and preparation method
InactiveCN103446617AWide range of antibacterialGood effectAbsorbent padsBandagesWound dressingFreeze-drying
The invention discloses a gentamicin sulfate / gelatin microsphere complex-loaded silk fibroin scaffold and a preparation method, belonging to the technical field of high molecular materials. The preparation method comprises the following steps: dropwise adding gentamicin sulfate solution with concentration of 10-50 mg / ml into gelatin microspheres, placing overnight at the temperature of 4-25 DEG C and then performing freeze drying to obtain a gentamicin sulfate / gelatin microsphere complex; adding the gentamicin sulfate / gelatin microsphere complex into 2-10 percent by weight of silk fibroin solution, uniformly stirring and then performing freeze drying to obtain the gentamicin sulfate / gelatin microsphere complex-loaded silk fibroin porous composite scaffold, wherein the porosity of the gentamicin sulfate / gelatin microsphere complex-loaded silk fibroin scaffold is 75-95 percent, the aperture of the gentamicin sulfate / gelatin microsphere complex-loaded silk fibroin scaffold is 50-200 mum, and the gentamicin sulfate / gelatin microsphere complex-loaded silk fibroin scaffold has long-acting antibacterial and drug slow-release functions and can be applied to the fields of skin wound healing and wound dressing.
Owner:JINAN UNIVERSITY
Natural compound feed additive for removing veterinary drug residues
InactiveCN104543404AAchieve productionGuarantee normal productionAnimal feeding stuffAstaxanthinVeterinary Drugs
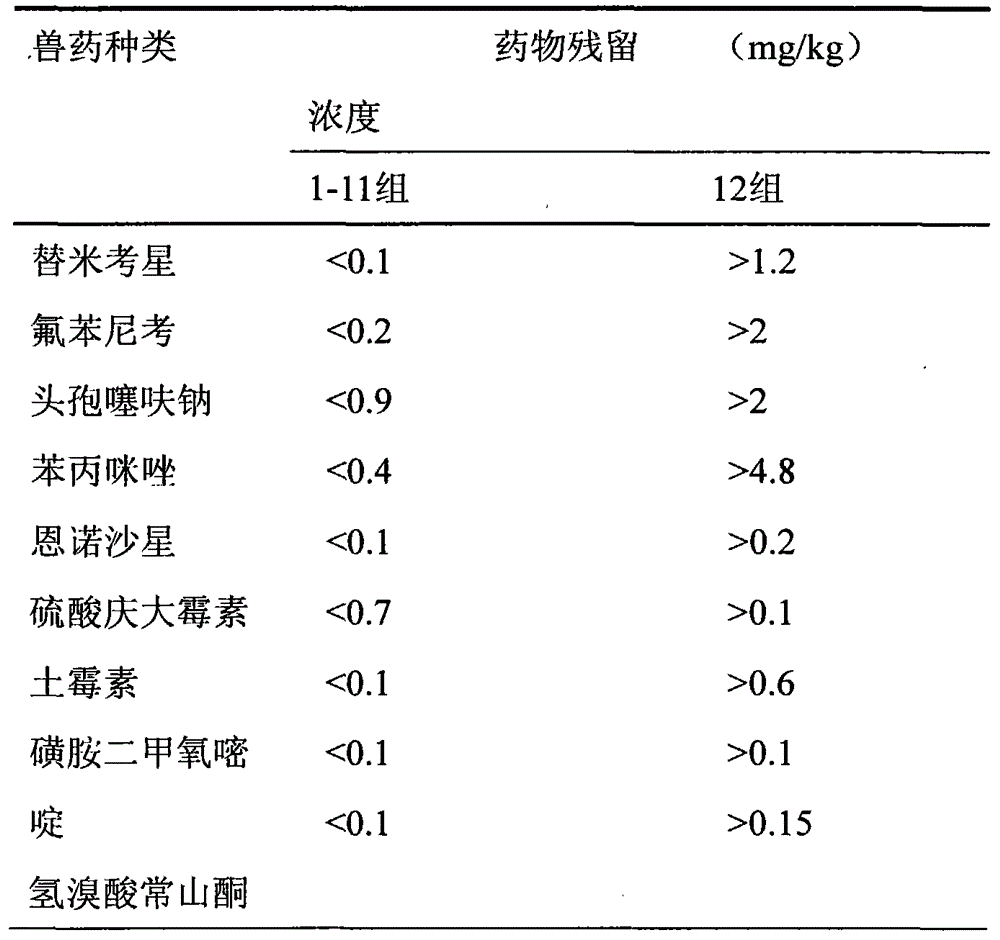
The invention discloses a natural compound feed additive for removing veterinary drug residues. The natural compound feed additive is prepared from the following components in parts by weight: 1 part of procyanidine, 1 part of astaxanthin, 1 part of resveratrol, 1 part of curcumin, 1 part of allicin, 1 part of porphyra polysaccharide, 1 part of porphyra polyphenol, 1 part of phycobiliprotein, 1 part of tea polyphenol, 1 part of quercetin, 1 part of chitosan and 1 part of tangeretin; and the ratio of the feed additive to the feed is (1:100) to (1:500) in use. According to the method, the residues of tilmicosin, florfenicol, ceftiofur sodium, thiabendazole, enrofloxacin, gentamicin sulfate, oxytetracycline, sulfadimethoxine and halofuginone hydrobromide in livestock or poultry bodies can be effectively reduced; the natural compound feed additive has the characteristics of being free of pollution and free of public hazard, and can be applied to removal of veterinary drug residues from food animals of which the veterinary drug residues exceed the standard due the factors such as veterinary drug application and environmental pollution, so as to ensure the hygiene and safety of animal food.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Gentamicin sulfate wrapped in polylactic acid/nano-hydroxyapatite composite microspheres and preparation method thereof
InactiveCN101791407AImprove adsorption capacityHigh drug loadingAntibacterial agentsOrganic active ingredientsApatiteMicrosphere
The invention relates to a gentamicin sulfate wrapped in polylactic acid / nano-hydroxyapatite composite microspheres, which comprises the following components in parts by weight: 1-10 parts of nano-hydroxyapatite, 1 part of gentamicin sulfate, 12.5-25 parts of polylactic acid and 1-17 parts of methyl cellulose. The invention further discloses a preparation method. The prepared gentamicin sulfate has the advantages of high drug loading, high entrapment efficiency, long-term sustained release and biodegradability.
Owner:SCHOOL OF OPHTHALMOLOGY & OPTOMETRY WENZHOU MEDICAL COLLEGE
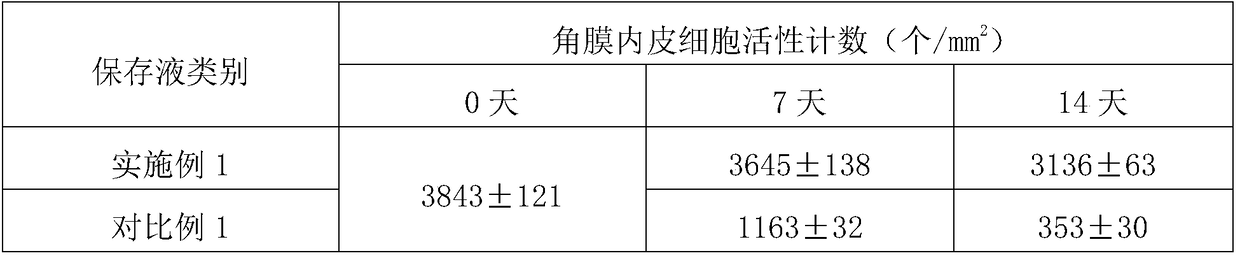
Corneal metaphase preserving liquid containing recombinant human serum albumin and preparation method thereof
InactiveCN109329274AMaintain structureMaintain transparencyDead animal preservationSodium bicarbonateGlutamine
The invention discloses corneal metaphase preserving liquid containing recombinant human serum albumin and a preparation method thereof. The corneal metaphase preserving liquid containing recombinanthuman serum albumin contains glutamine, calcium pantothenate, choline chloride, folic acid, inositol, nicotinamide, pyridoxal.HCl, riboflavin, thiamine.HCl, KCl, NaCl, NaH2PO4.2H2O, glucose, dextran 40, sodium pyruvate, vitamin C, chondroitin sulphate sodium, sodium hyaluronate, non-animal recombinant human serum albumin, gentamicin sulfate, sodium bicarbonate, piperazine-1-erhanesulfonic acid, phenol red indicator, and water for injection. The non-animal recombinant human serum albumin is added to replace serum to improve corneal endothelial cell survival rate; and then the non-animal recombinant human serum albumin cooperates with a compound system of the chondroitin sulphate sodium, the sodium hyaluronate and the dextran 40, the survival rate of the corneal endothelial cell is greatly ensured.
Owner:镇江雷音再生医学科技有限公司
Disinfectant for primary cell culture of animals and preparation method of disinfectant
InactiveCN108184890ABroad spectrum antibacterialGrowth inhibitionBiocideFungicidesPenicillinDisinfectant
Owner:CHINA JILIANG UNIV
Preparation method for decreasing impurities of gentamicin sulfate
InactiveCN107779486ALess impuritiesSimple production processSugar derivativesSugar derivatives preparationGlycineSpore
The invention discloses a preparation method of gentamicin sulfate capable of reducing impurities, which can significantly reduce the impurities of gentamicin sulfate products, meets the requirements of "Chinese Pharmacopoeia" (2015 edition), and the production process is simple and easy to operate; The present invention differs from the prior art in the following three points: 0.001% adenosine triphosphate and 0.001% glycine are added to the spore slant culture medium; 0.001% adenosine triphosphate and 0.001% glycine are added to the seed culture medium; 0.003% adenosine triphosphate is added to the fermentation medium and 0.003% glycine; the total miscellaneous content of the gentamicin sulfate product produced before the improvement > 4%, and sisomicin > 1.5%; Somicin <1.5%, Minor Normycin (Small Normicin) <3%.
Owner:福安药业集团烟台只楚药业有限公司
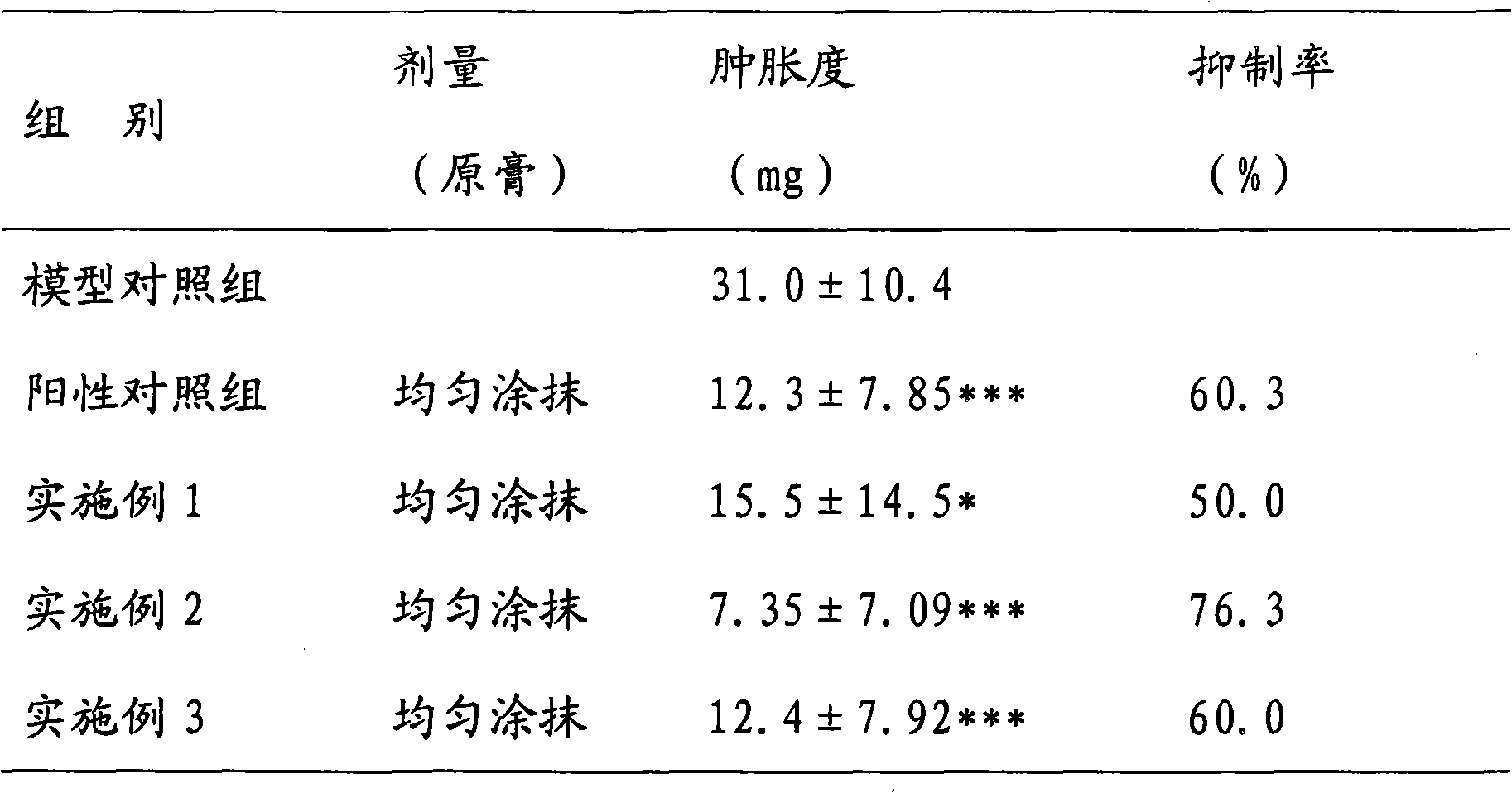
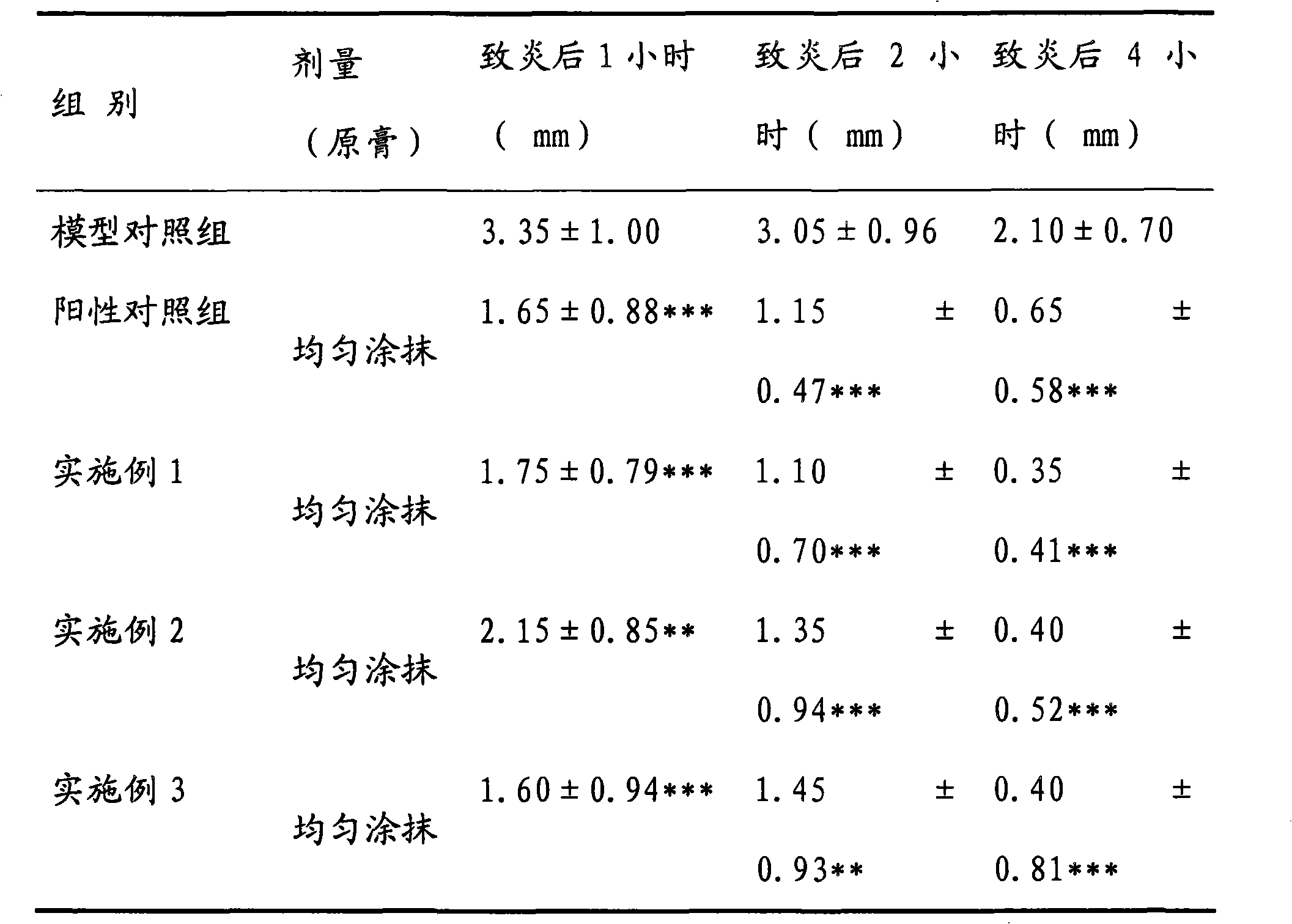
Absorption-promoting pharmaceutical composition ointment for treating skin diseases and preparation method thereof
InactiveCN102172355ASignificant effectNo obvious side effectsOrganic active ingredientsAerosol deliveryDiseaseSide effect
The invention discloses a preparation method of a pharmaceutical composition ointment for treating skin diseases. The pharmaceutical composition contains the following components in proportion by weight: 0.05-0.08% of betamethasone valerate, 0.08-0.12% of chlorocresol and 0.08-0.12% of gentamicin sulfate. The pharmaceutical composition ointment is mainly used for treating dermatitis, eczema, contact dermatitis, seborrheic dermatitis, photodermatitis, neurodermatitis, intertrigo, exfoliative dermatitis, skin itch, psoriasis, first-degree burn and the like. The pharmaceutical composition ointment has the characteristics of jointly applying pharmaceutical components with anti-inflammatory, antifungal and antibacterial curative effects, playing the curative effects in multiple aspects, overcoming the defect that an individual component can not more effectively treat skin mycotic infection, skin allergy, dermatitis, eczema and the like, or concurrence of mycotic infection, skin allergy, dermatitis, eczema and the like, and relieving the serious side effects caused by singly using a big amount of hormone for treating dermatitis, eczema and the like; and a component for promoting percutaneous absorption is added to auxiliary materials to enhance the pharmaceutical curative effect and promote absorption. The ointment disclosed by the invention has the advantages of exact efficacy, strong local pertinence, no irritation on skin, and the like, and the preparation method of the ointment is suitable for industrial production.
Owner:北京凯宾鸿生物医药科技有限公司 +1
Diluting powder for artificial fertilization of pigs
The invention provides diluting powder for artificial fertilization of pigs. The diluting powder is prepared by mixing the following raw materials in parts by weight: 27-28 parts of glucose, 6.5-7.5 parts of sodium citrate, 0.5-1.5 parts of sodium bicarbonate, 2-3 parts of ethylene diamine tetraacetic acid disodium, 2.5-3.5 parts of anhydrous citric acid, 5-6 parts of tris(hydroxymethyl)aminomethane, 0.1 part of gentamicin sulfate, 0.1g of kanamycin and 1000 parts of distilled water. The diluting powder has low cost and good effects.
Owner:HENAN RUIXIANG AGRI & ANIMAL HUSBANDRY
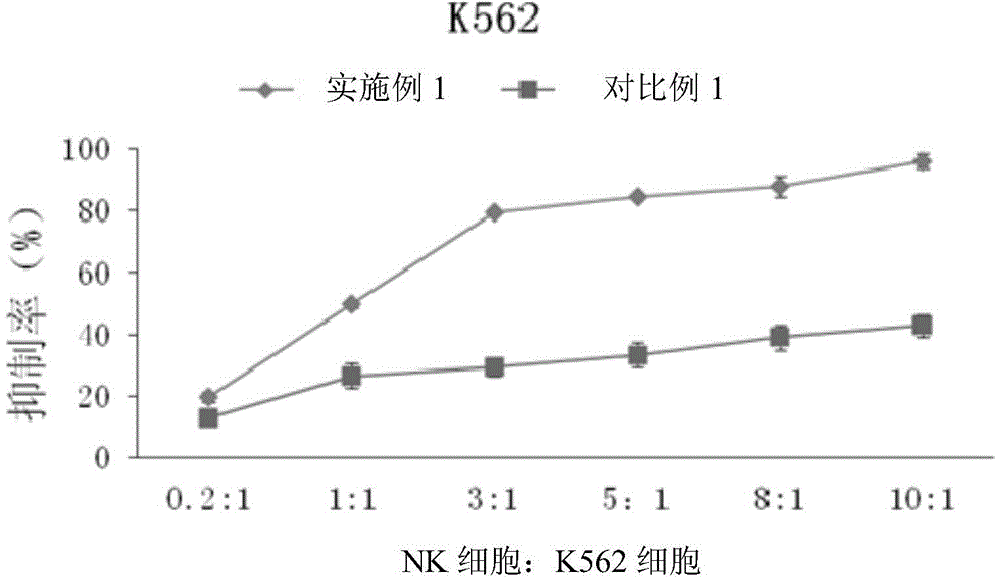
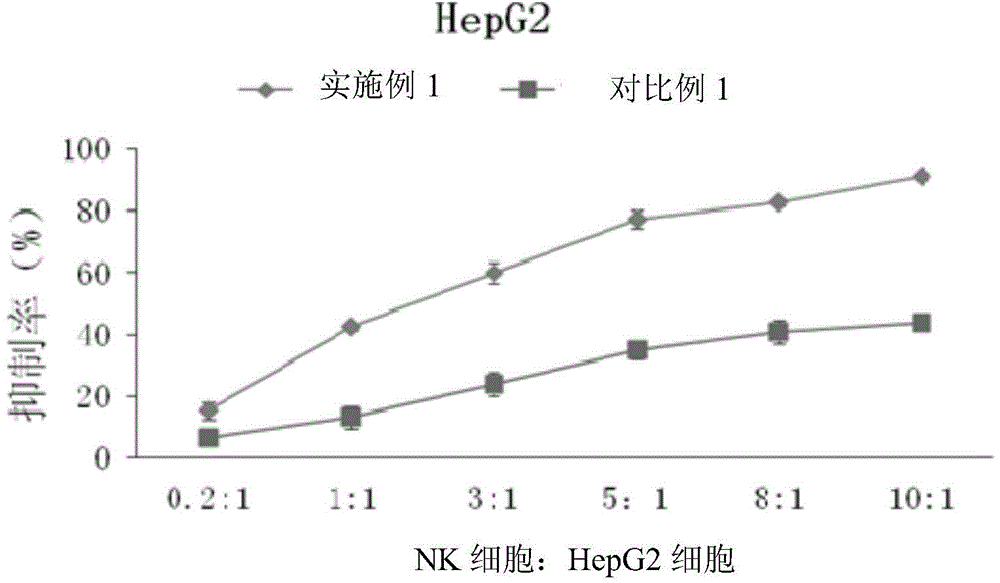
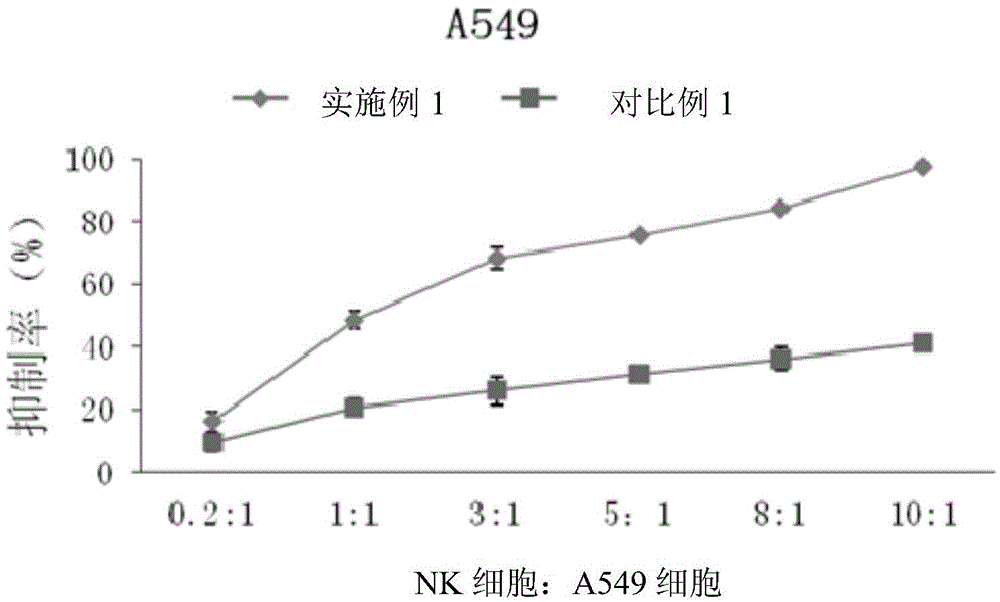
Method for proliferating natural killer cells and culture medium composition
The invention discloses a method for proliferating natural killer cells. The method comprises the following steps: (1) separating lymphocytes from whole blood; inoculating the lymphocytes in a first culture medium for first proliferation culture to obtain a first proliferation culture product; (2) mixing the first proliferation culture product with a second culture medium according to a volume ratio of 1 to (0.5-2) and performing second proliferation culture to obtain a second proliferation culture product; (3) replacing the first proliferation culture product with the second proliferation culture product, and repeatedly operating according to step (2) for 2-7 times. The invention also provides a culture medium composition. The culture medium composition contains a basal culture medium, L-glutamine, gentamicin sulfate, mannatide, interleukin 2 and interleukin 15. The proliferation method disclosed by the invention is a natural killer cell proliferation method which is simple, convenient, high in efficiency, easy to operate and relatively high in safety.
Owner:HANGZHOU S EVANS BIOSCI LTD
External medicine for treating psoriasis and medicine composition
The invention discloses an external medicine for treating psoriasis. The external medicine for treating psoriasis is in a cream type, and is characterized by being prepared from the following components in parts: 30 to 42 parts of centipede, 20 to 25 parts of scorpion, 5 to 7 parts of borneol, 400 to 600 parts of vaseline, and 30 to 40 parts of gentamicin sulfate. The invention also provides a medicine composition for treating the psoriasis.
Owner:曾仲强
Granular cell removing solution and preparation method thereof
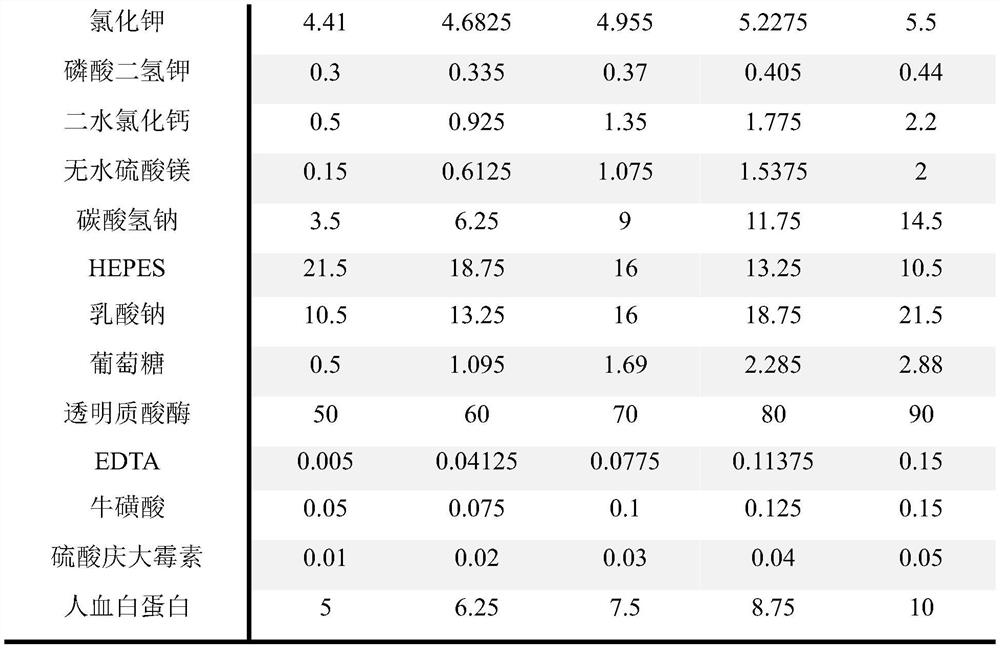
The invention belongs to the technical field of human assisted reproduction, and relates to a granular cell removing solution of human oocyte. The solution is prepared by dissolving a proper amount ofEDTA and taurine in a basic salt solution containing a granular cell removing enzyme. The content of the EDTA is 0.005 to 0.015 mM / L. The content of the taurine is 0.05 to 0.15 mM / L. The granular cell removing enzyme is hyaluronidase, and the content of the granular cell removing enzyme is 50-90 IU / mL. The basic salt solution comprises the following components: 90.08 to 101.5 mM / L of sodium chloride, 4.41 to 5.50 mM / L of potassium chloride, 0.50 to 2.20 mM / L of calcium chloride dihydrate, 0.30 to 0.44 mM / L of potassium dihydrogen phosphate, 0.15 to 2.00 mM / L of magnesium sulfate heptahydrate,3.50 to 14.50 mM / L of sodium bicarbonate, 10.5 to 21.5 mM / L of 4-hydroxyethylpiperazine ethane sulfonic acid (HEPES), 0.01 to 0.05 g / L of gentamicin sulfate, 0.50 to 2.88 mM / L of glucose, 0.31 to 0.35 mM / L of sodium pyruvate, 10.5 to 21.50 mM / L of L-sodium lactate and 5 to 10 g / L of human serum albumin.
Owner:SHENZHEN VITAVITRO BIOTECH CO LTD
Hepatitis B virus serum sample preserving diluent and preparation method thereof
InactiveCN108998569AUndamagedFew ingredientsMicrobiological testing/measurementMicroorganism based processesSerum igeSerum samples
The invention discloses a hepatitis B virus serum sample preserving diluent. The hepatitis B virus serum sample preserving diluent comprises Trise-HCL, NaCL, BSA, gentamicin sulfate, P-300, sunset yellow, lemon yellow and deionized purified water. On the basis of 1 L of the deionized purified water, the contents of Trise-HCL, NaCL, BSA, gentamicin sulfate, P-300, sunset yellow and lemon yellow are0.01 mol, 8.8 g, 20 g, 0.68 ml, 2.0 ml, 0.01 g, and 0.01 g, respectively. The hepatitis B virus serum sample preserving diluent of the invention has the advantages that the diluent comprises few components; the preparation method is simple; the prepared finished preserving diluent can protect the structure of a viral nucleic acid material from damage, inhibit the activity of DNase and effectivelyprotect the DNAs of a virus from degradation, has the advantages of stability, easy storage, convenience, etc., and is applicable to preservation and dilution of the hepatitis B virus (HBV DNA) serumsample. Test results prove that after addition of the preserving diluent prepared by the invention into the hepatitis B virus (HBV DNA) serum sample, DNAs and RNAs can be completely stored at 37 DEGC for 1 week, stored at 20-25 DEG C for 1 month, stored at 4 DEG C for 6 months and store at -20 DEG C or -80 DEG C for a long time.
Owner:AUTOBIO DIAGNOSTICS CO LTD
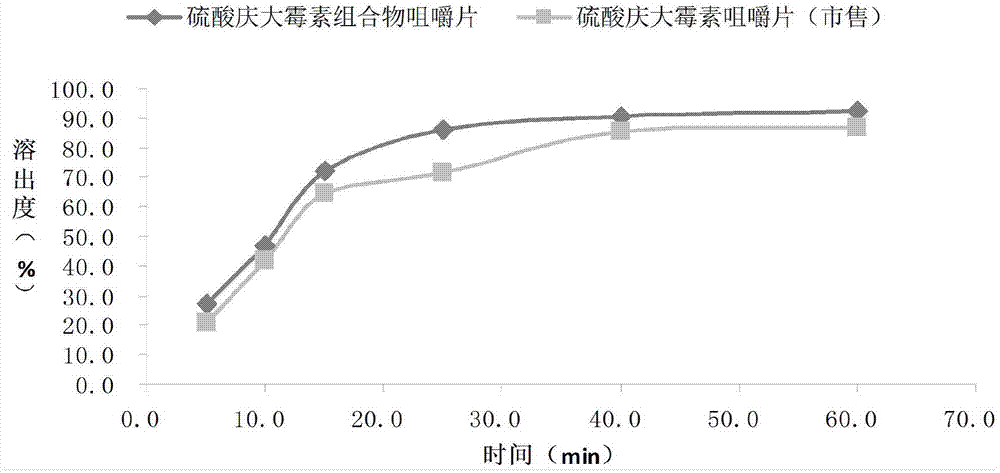
Gentamicin sulfate composition chewable tablets and preparation method thereof
InactiveCN104490820AEasy to operateSimple prescriptionAntibacterial agentsOrganic active ingredientsSucroseDrugs preparations
The invention provides gentamicin sulfate composition chewable tablets and a preparation method thereof, and relates to the technical field of medicine and medicine production. The gentamicin sulfate composition chewable tablets comprise gentamicin sulfate, starch and sucrose; the tablets overcome the shortcomings of common chewable tablets, and the types and amount of accessories in the gentamicin sulfate chewable tablets are reduced; and the medicine preparation has good performance, high bioavailability and good stability, is highly accepted by patients, avoids gritty feel and has an important value in clinical application.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Novel medicament for treating bacterial septicemia of freshwater fish
InactiveCN103933050AEasy to useSimple preparation processAntibacterial agentsOrganic active ingredientsGlycineVitamin C
The invention discloses a novel medicament for treating bacterial septicemia of freshwater fish. The novel medicament comprises the following components in parts by weight: 30-60 parts of gentamicin sulfate for oral administration, 1-3 parts of vitamin E, 0.2-0.4 part of vitamin C, 0.3-0.6 part of glycine, 0.2-0.4 part of tyrosine, 1-3 parts of enrofloxacin, and 300-600 parts of water. Compared with the prior art, the novel medicament has the beneficial effects of excellent usage effect, simple preparation technology, low cost and the like.
Owner:黎作民
Method for measuring histamine content in gentamicin sulfate for veterinary use
ActiveCN110632214AOvercome extraction difficultiesMake up for quantitative detection vacanciesComponent separationNitrogenAcetonitrile
The invention discloses a method for measuring histamine content in gentamicin sulfate for veterinary use, and belongs to the technical field of analytical chemistry. The measuring method uses hydrochloric acid-methanol solution or hydrochloric acid-acetonitrile solution to extract histamine of diluted gentamicin sulfate for veterinary use, purifies impurities in the extracting solution with C18 powder, re-dissolves by nitrogen blowing and acetonitrile formic acid solution, and then performs LC-MS / MS determination. The method for measuring histamine content in gentamicin sulfate for veterinaryuse provided by the invention enables the HPLC mass spectrometer to better exclude matrix interferences by pre-processing the sample, and then uses LC-MS / MS to quickly and accurately perform detection and quantification of histamine in gentamicin sulfate for veterinary use, which overcomes the difficulty in extracting histamine in gentamicin sulfate for veterinary use, and makes up for the inability to quantitative detection of histamine content in gentamicin sulfate for veterinary use in the prior art.
Owner:INSPECTION & QUARANTINE TECH CENT OF FUJIAN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Culture medium for preparing endometrial stem cells as well as preparation method
InactiveCN109456937AImprove survival ratePromote application developmentCell dissociation methodsCulture processPenicillinDigestion
The invention relates to the technical field of tissue culture, and discloses a culture medium for preparing endometrial stem cells as well as a preparation method. On the basis of a serum-free culture medium, Pall serum substitute, penicillin / streptomycin, gentamicin sulfate, glutamine, MEM NEAA, EGF, bEGF, sodium pyruvate and VEGF are added as nutritional ingredients of the endometrial stem cells, so that the survival rate of the endometrial stem cells can be significantly improved; and by combining with the mixed digestion of collagenase I and Dispase II, the survival rate of the endometrial stem cells can be further improved, and the development application of the endometrial stem cells can be promoted.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Gentamicin sulfate prepared through enhanced microbial fermentation, and application method of gentamicin sulfate
ActiveCN113403237AHigh purityIncrease production capacityBacteriaMicroorganism based processesBiotechnologyMicroorganism
The invention claims to protect gentamicin sulfate prepared through enhanced microbial fermentation, and an application method of the gentamicin sulfate. The enhanced microbial engineering bacterium is a Micromonospora purpurea C2 1591 strain which is obtained by screening through a normal-pressure room-temperature plasma mutagenesis technology, and the Micromonospora purpurea C2 1591 strain is registered and collected in the China General Microbiological Culture Collection Center (CGMCC for short, the address is No.3, No.1 Yard, Beichen West Road, Chaoyang District, Beijing) on June 13, 2021, and the collection number is CGMCC No.59450. The screened engineering bacterium for directionally producing gentamicin C2 in high yield can directionally produce the gentamicin C2 in the high yield, the component ratio of gentamicin C2 is increased to 85% or above from the original about 15%, and the highest fermentation titer of an optimized formula is 1184 U / mL through Plackett-Burman design and is increased by 42% than the titer of an original formula. Compared with an original strain, the yield of the gentamicin C2 is increased by three times, in addition, other isomers or impurities are hardly contained, the gentamicin C2 can be directly applied downstream, complex biotransformation and purification is not required, a large amount of cost is saved, and an industrialization requirement is met.
Owner:青岛安惠仕生物制药有限公司
Dragon fish white-eye disease treatment liquid and preparation method thereof
InactiveCN105168318AEnhanced inhibitory effectReduce infectionAntibacterial agentsOrganic active ingredientsNitrofurazoneAdemetionine
The invention relates to a dragon fish white-eye disease treatment liquid, which is characterized by comprising the following components in parts by weight: 1 to 2 parts of gentamicin sulfate, 3 to 5 parts of ethyl paraben, 5 to 8 parts of nitrofurazone, 5 to 10 parts of bendazac lysine, 5 to 10 parts of sodium chloride (NaCl), and 20 to 30 parts of traditional Chinese medicine liquid, wherein the traditional Chinese medicine liquid is prepared by boiling the following traditional Chinese herbals in water (50 to 60 parts by weight): 2 to 5 parts of cassia seed, 2 to 4 parts of wild chrysanthemum, 3 to 4 parts of inula flower, and 1 to 2 parts of Chinese milk vetch. The treatment liquid is prepared by adding gentamicin sulfate, ethyl paraben, nitrofurazone, bendazac lysine, and NaCl into the traditional Chinese medicine liquid and evenly mixing.
Owner:TAIXING HEQING MACHINERY FITTINGS FACTORY
Sample diluent applicable to peripheral blood detection
InactiveCN107102134ARaw materials are easy to getAvoid the risk of contracting certain virusesMaterial analysisAdditive ingredientProclin
The invention discloses a sample diluent applicable to peripheral blood detection. The sample diluent per 1,000ml is prepared from the ingredients: 20.5g of glucose, 8.5g of sodium chloride, 2g of bovine serum albumin, 0.55g of citric acid, 0.4ml of Proclin 300 and 2ml of gentamicin sulfate. The sample diluent is readily available in required raw materials, low in price and free of blood serum ingredient, the risk of infecting some special viruses is avoided, the osmotic pressure of solutions inside and outside cells is balanced by various ions, and the preservation effect is remarkable. The optimized sample diluent is high in applicability, wide in range of application and superior in oxidation resistance and can exert a protecting action during the transportation and preservation of peripheral blood diluted samples.
Owner:江苏华冠生物技术股份有限公司
Preparation method of anti-Mullerian hormone quality control substance
InactiveCN109342746AReduce matrix effectCommercially availableBiological testingMedicineFreeze-drying
The invention discloses a preparation method of an anti-Mullerian hormone quality control substance. The preparation method comprises the following steps: selecting hormone removing blood serum meeting the demand; adding Proclin300, gentamicin sulfate, sodium azide, thiomersalate and trehalose to be uniformly mixed; first, measuring a background value of the anti-Mullerian hormone; then adding ananti-Mullerian hormone positive substance to the set value 0.5-20.0 ng / mL; and sub-packaging the substance obtained in the S4 in a specification of 1.0 ml / bottle in a 4 ml brown glass bottle for vacuum freezing and drying to obtain the anti-Mullerian hormone quality control substance and storing the anti-Mullerian hormone quality control substance at 2-8 DEG C. The quality control substance selects the hormone removing blood serum and can be in commercial production. Meanwhile, as the anti-Mullerian hormone quality control substance is high in consistence to a normal human sample, the matrix effect is reduced to the maximum extent, so that the anti-Mullerian hormone quality control substance is suitable for instruments and reagents of mainstream factories. The quality control substance isa freeze-dried quality control substance which is high in stability, is not affected by factors such as transportation and temperature, and is stable in performance.
Owner:郑州标源生物科技有限公司
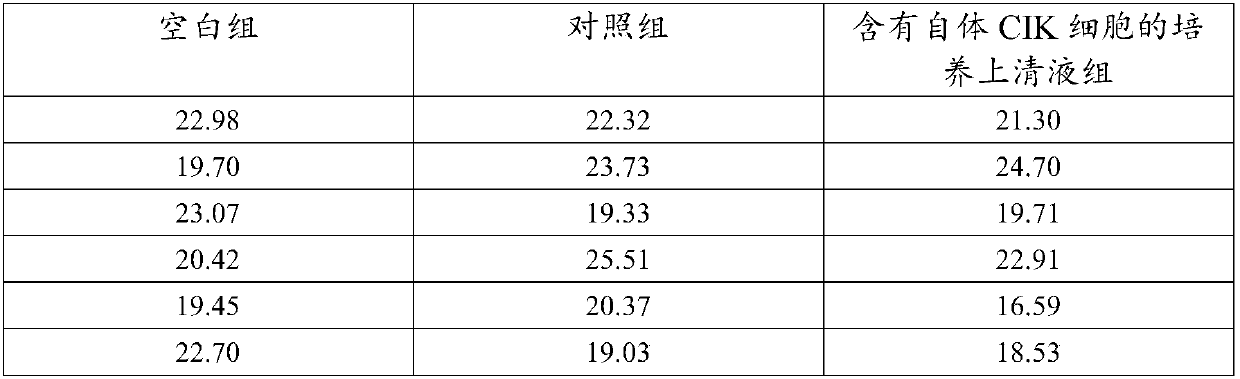
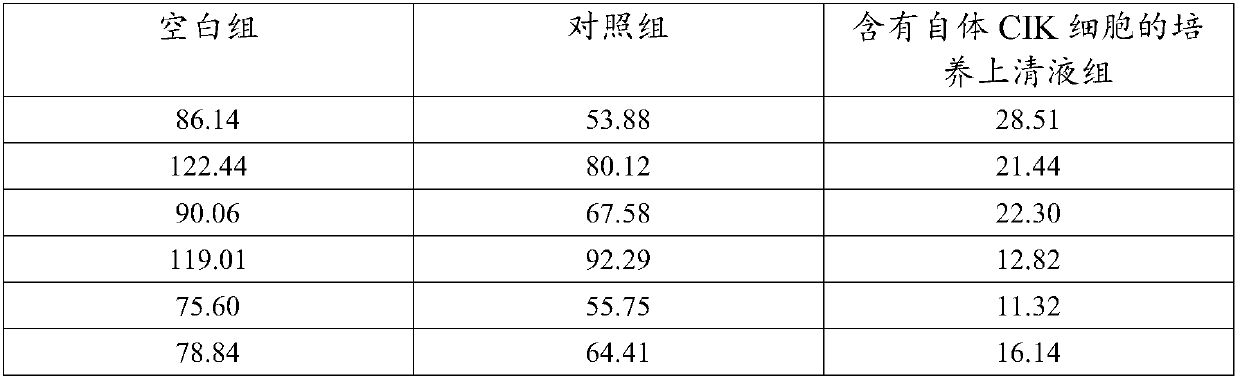
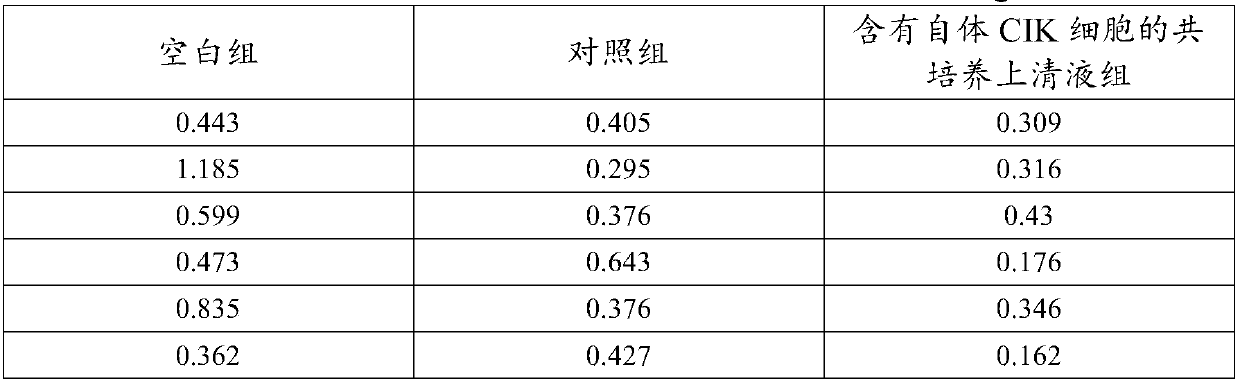
Co-culture supernatant containing autologous CIK cells and application thereof
InactiveCN107779435AImprove anti-tumor effectMaintain anti-tumor effectMammal material medical ingredientsBlood/immune system cellsCentrifugationHuman albumin
The invention discloses co-culture supernatant containing autologous CIK cells and application thereof, wherein the co-culture supernatant containing the autologous CIK cells is prepared by a following method. The specific method comprises the steps that: (1) the autogenous CIK cells are cultured until the fourteenth day, and concentration of the autologous CIK cells reaches 1-5*10<6> cells / ml, and after centrifugation, supernatant 1 and bottom cells 1 are obtained respectively; (2) the bottom cells 1 are washed with normal saline and centrifuged to obtain bottom cells 2; (3) the supernatant 1obtained in step (1) is further centrifuged, and bottom flocculent is discarded to obtain supernatant 2; (4) the supernatant 2 of the step (3) and the bottom cells 2 of the step (2) are mixed, addedwith the normal saline, human albumin, and gentamicin sulfate to prepare cell suspension, namely, the co-culture supernatant containing autologous CIK cells. Natural cytokines synthesized and secretedby the autologous CIK cells are more effective in enhancing immune function, resolving myelosuppression and immunosuppression, and improving anti-tumor effects of the autologous CIK cells.
Owner:广州市金航生物科技有限公司
Quality control product suitable for quantitative detection of tumor marker Pro-GRP
InactiveCN109342728AImprove uniformityImprove stabilityReference solutionsMatrix solutionFreeze-drying
The invention discloses a quality control product suitable for quantitative detection of a tumor marker Pro-GRP, comprising the following steps of: first, selecting a de-hormone serum suitable for thetumor marker Pro-GRP, and then storing at 2-8 DEG C after thawing; successively adding 0.1-0.5% ADP, Proclin300, gentamicin sulfate, and 1%-10% trehalose to an matrix solution and storing at 2-8 DEGC; adding a tumor marker Pro-GRP antigen into the matrix solution, and then adding lauryl sodium sulfate to obtain the quality control product of a liquid tumor marker Pro-GRP; and dispensing the product in a brown glass bottle, obtaining a finished product of the quality control product of the tumor marker Pro-GRP after performing vacuum freeze drying, and storing the finished product in a refrigerator at 2-8 DEG C. The quality control product of the tumor marker Pro-GRP prepared by the invention has good uniformity and stability, is not affected by transportation, temperature and the like, and can be applied to quantitative detections of different instrument models and different reagents. The quality control product is a lyophilized product, and has reliable quality and high accuracy. The raw materials for preparing the quality control product are widely used, the preparation method is simple, and the cost is low.
Owner:郑州标源生物科技有限公司
Sperm refrigerating fluid for human assisted reproduction and preparation method thereof
The invention discloses sperm refrigerating fluid for human assisted reproduction and a preparation method thereof. The cane sugar in the sperm refrigerating fluid is replaced trehalose. The sperm refrigerating fluid comprises 95.00-105.00 mmol / L of sodium chloride, 3.45-6.55 mmol / L of potassium chloride, 0.55-0.65 mmol / L of magnesium sulfate, 1.75-2.22 mmol / L of calcium chloride, 0.18-0.39 mmol / Lof sodium dihydrogen phosphate, 0.58-1.78 mmol / L of sodium citrate, 3.85-5.45mmol / L of sodium bicarbonate, 15.00-25.00mmol / L of HEPES and MOPS, 0.38-0.68 mmol / L of glucose, 0.28-0.42 mmol / L of sodium pyruvate, 12.48-23.68 mmol / L of sodium lactate, 5-15 mg / mL of human serum albumin, 7-14 [mu]g / mL of gentamicin sulfate, 25-35% (v / v) of glycerin, and 0.15-0.65 mol / L of trehalose. The preparation method comprises the following steps: weighing various components, dissolving the components in injection-grade water, performing filtering and sterilizing by using a 0.2 [mu]m filter membrane, performing sampling and testing, wherein the pH value is controlled to be 7.00-7.40, the osmotic pressure ranges from 1720 mOsm / Kg to 2020 mOsm / Kg, the bacterial endotoxin is less than 0.25 EU / ml, and the blastocyst formation rate is greater than or equal to 80% when 1 cell mouse embryo is cultured for 96h.
Owner:东蕴医疗科技(上海)有限公司
A kind of hepatitis C virus antigen-antibody combined detection kit and preparation method thereof
A hepatitis c virus antigen-antibody joint detection reagent box is characterized by comprising a calibrator(1), a double-marker enzyme conjugate (2), a negative and positive contrast (3), light-emitting liquid (4) and a micropore coated plate (5), wherein the light-emitting liquid contains light-emitting liquid 1 and light-emitting liquid 2, the light-emitting liquid 1 contains luminal 0.7 g / L, cinnamic acid 0.9 g / L, 4-iodophenylboronic acid 0.2 g / L, iodobiphenol 0.25 g / L, dimethylformamide 25 ml / L, polyving akohol 5 g / L, polyvinylpyrrolidone 8 g / L, polyethylene glycol 600 3 g / L, ethylenediamine tetraacetic acid 4 g / L, gentamicin sulfate 1600 thousands / L, urea peroxide 0.4 g / L, and pH 9.0 Tris buffer solution 0.1 mol / L. The light-emitting liquid 2 contains acridinium ester derivative 0.1 mg / ml, polyethylene glycol 600 3 g / L and 0.1 mol / L of pH 9.0 Tris buffer solution containing 0.1% of TWEEN-20. The invention further discloses a preparation method and a using method of the reagent box. The hepatitis c virus antigen-antibody joint detection reagent box has the advantages of being quick in reaction and low in cost.
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
Medicinal composition for treating infant oral ulcer
InactiveCN103181946ALittle side effectsGood curative effectDigestive systemHeterocyclic compound active ingredientsSide effectVitamin C
The invention belongs to the technical field of medicine, and discloses a medicinal composition for treating infant oral ulcer. The medicinal composition is prepared from gentamicin sulfate, prednisone acetate, vitamin C, vitamin B1, vitamin B2, niacinamide, vitamin B6 and watermelon frost as raw materials according to different characteristics of the component medicinal raw materials and proportion. The raw materials are dried at low temperature, porphyrized, mixed well, sterilized and so on to prepare a powder agent or a spraying agent. The medicinal composition is characterized by new formula, convenience in preparation and obvious effect. The raw materials for preparing the drug are wide in source of feed and low in cost, so that the cost of the drug is reduced. Clinically proved by 200 more cases of infant oral ulcer patients, the efficiency rate is up to 100%, and no special toxic and side effect is shown.
Owner:齐凤芹
Skin mucous membrane damage antibacterial disinfectant and preparation method thereof
InactiveCN107737131AThe formula is scientific and simpleEfficient killingAntibacterial agentsBiocideDisinfectantLotion
The invention provides a skin mucous membrane damage antibacterial disinfectant and a preparation method thereof. The skin mucous membrane damage antibacterial disinfectant is mainly prepared from vitamin 12 and gentamicin sulfate, is capable of effectively killing various bacteria and harmful microorganisms of skin mucous membranes, is applied to antibacterial disinfection on radioactive and non-radioactive mucous membranes, is also applicable to antibacterial sterilization and disinfection for normal and damaged skin, mucous membrane tissue, hygiene and disease control and the like, and hasfunctions of disinfection and bacterial resistance on radioactive combined injury. The disinfectant can be a spray, powder, a solution, gel and an ointment associated with medical preparations, and can be also made into sanitary products such as lotions, wet tissue paper, facial masks, mouth-muffles, filming agents and toothpaste products.
Owner:杨小玉
Compound lincomycin hydrochloride injection for livestock and preparation method thereof
InactiveCN101411719AReasonable compositionEasy to makeOrganic active ingredientsAntiinfectivesBerberineCurative effect
The invention relates to a compound lincomycin hydrochloride injection for livestock. The compound lincomycin hydrochloride injection is characterized in that the compound lincomycin hydrochloride injection is prepared through evenly mixing the following compositions in weight portion: 8 to 20 portions of lincomycin hydrochloride, 5 to 20 portions of berberine bisulfate, 5 to 15 portions of gentamicin sulfate-micronomicin sulfate and 45 to 100 portions of water for injection. The preparation method comprises the following steps: (1) according to weight portion, 8 to 20 portions of the lincomycin hydrochloride, 5 to 20 portions of the berberine bisulfate and 5 to 15 portions of the gentamicin sulfate-micronomicin sulfate are weighed; (2) the lincomycin hydrochloride is put into 30 to 40 portions of the water for injection at a temperature of between 45 and 55 DEG C and is stirred for 10 to 25 minutes to clarification; (3) 10 to 20 portions of the water for injection is taken out, is sequentially added with 5 to 15 portions of the gentamicin sulfate-micronomicin sulfate and 5 to 20 portions of the berberine bisulfate and is stirred and dissolved; and (4) a solution prepared in the step 2 and a solution prepared in the step 3 are mixed, are evenly stirred and are kept for 15 to 20 minutes; and after a medicine solution is clarified, the residual part of the water for injection is added into the medicine solution and is evenly stirred and filtered. The compound lincomycin hydrochloride injection has a reasonable formula, simple preparation and remarkable curative effect.
Owner:TIANJIN SHENGJI GRP CO LTD
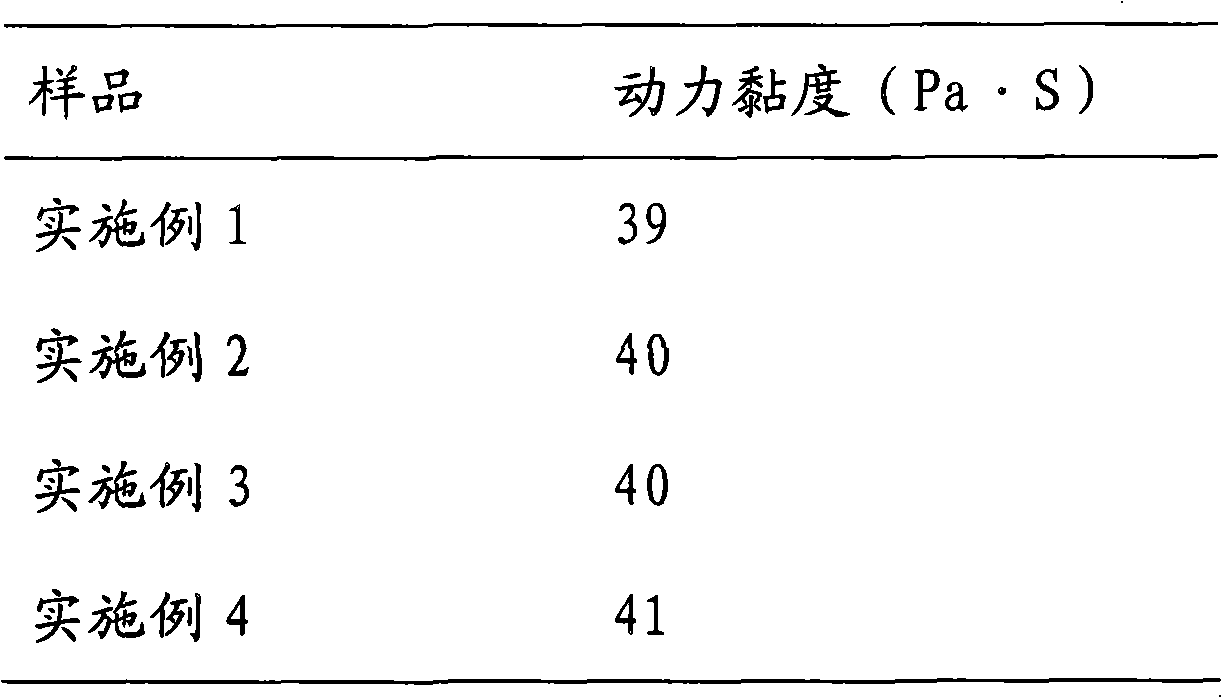
Preparation Technology of Compound Gentamicin Membrane
ActiveCN106344604BPromote healingInhibitory activityAntibacterial agentsOrganic active ingredientsDexamethasone acetateDissolution
The invention discloses a preparation process of a compound gentamicin film, made from, according to the formula, 750-770 parts of gentamicin sulfate, 2000-2100 parts of tetracaine hydrochloride, 50-60 parts of dexamethasone acetate, suitable polyvinyl alcohol (PVA), suitable chitosan, suitable sodium hydrogen sulfite, suitable steviosin, suitable tartaric acid, suitable indigo, and suitable of lemon yellow; the compound gentamicin film is prepared through the preparation of a drug film, the preparation of tetracaine hydrochloride hydroxypropyl-Beta-cyclodextrin inclusion, and the preparation of dissolution and mixing of other materials. By using the process of the invention, gel pharmaceutical value in the formula is made full use, effective ingredients of tetracaine hydrochloride tend to be more stable in the drug film and are better utilized, and the compound gentamicin film has low loss of effective ingredients and stable quality.
Owner:FUZHOU JINXIANG CHINESE MEDICINE PHARMA
Gastritis treating tablet
InactiveCN1093402CEnhanced inhibitory effectMoisture proofDigestive systemCarbohydrate active ingredientsCelluloseCarboxymethyl starch
The tablet for treating gastrilis features that genetamiycin sulfate, novocainum and vitamin B12 are compounded into compound preparation, and each tablet contains gentamicin sulfate 20000-4000 IU, novocainum 0.1-0.2g and vitamin B12 50-100 mirograms. During the preparation, starh and carboxymethyl starch are used as pelletizing supplementary materials, magnesium stearace is used as tabletting flow assistant, and gastro-soluble acrylic resin and hydroxyproyl cellulose are used as main supplementary materials for quick gastro-soluble membranous. The tablet of the present invention has determinate curative effect.
Owner:苏乐群 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com