Hepatitis B virus serum sample preserving diluent and preparation method thereof
A hepatitis B virus and diluent technology, applied in biochemical equipment and methods, microorganism-based methods, and microbial determination/inspection, etc., can solve the problems of easy bleeding and degradation, poor preservation effect, etc. The effect of reducing and inhibiting DNase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of Hepatitis B Virus Serum Sample Preservation Diluent
[0025] The first step is to measure an appropriate amount of deionized purified water;
[0026] In the second step, add accurately measured Trise-HCL 0.01mol / L, NaCL 8.8g, BSA 20g, gentamicin sulfate 0.68ml, P-300 2.0ml, sunset yellow 0.01g, tartrazine 0.01g into the deionization purification water and stir well;
[0027] The third step is to adjust the pH of the solution to 7.8~8.2, then add deionized purified water to make the volume to 1L;
[0028] The fourth step is to seal and autoclave (121°C, 15min), then store at 2-8°C.
Embodiment 2
[0029] Example 2 Dilute clinical sample experiment
[0030] 1. Instruments, reagents and samples
[0031] Main instruments: dry constant temperature metal bath, FLEX extractor, biological safety cabinet, pipette, Hongshi real-time fluorescence quantitative PCR instrument, etc.
[0032] Main reagents: Hepatitis B virus nucleic acid assay kit (PCR-fluorescent probe method) from Sun Yat-Sen University Daan Gene Co., Ltd.
[0033] Sample: fresh HBV clinically positive sample.
[0034] 2. Implementation plan
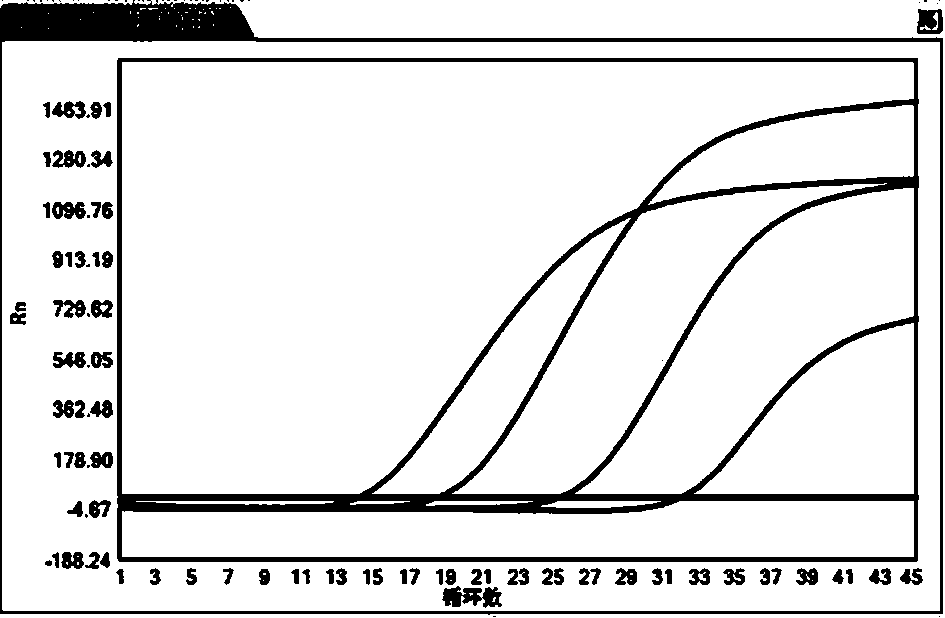
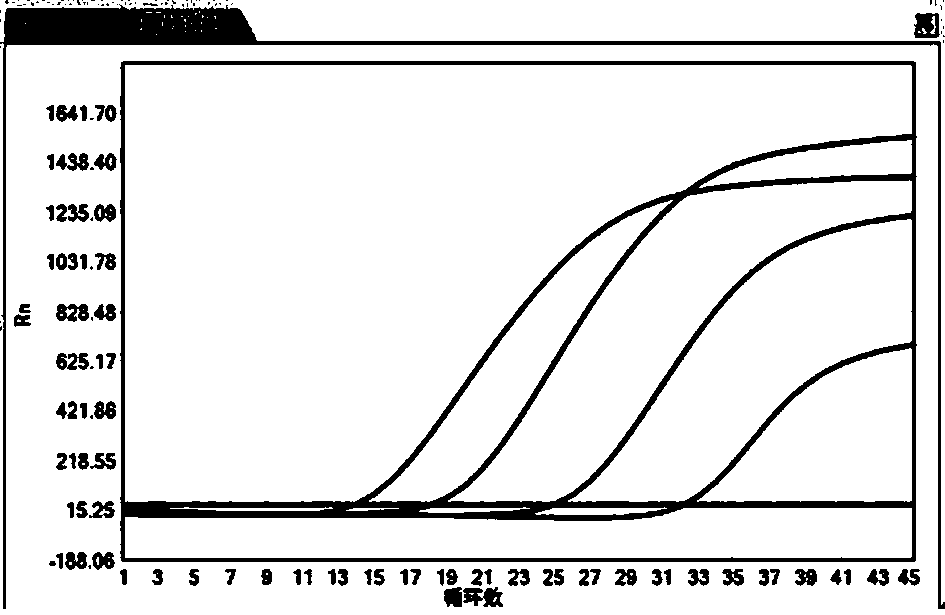
[0035] The virus serum sample preservation diluent prepared in Example 1 is used to dilute the HBV positive sample 5 times, 50 times, 500 times, 5000 times. Two dilutions of the control combination experimental group were designed, and the samples diluted in two different dilutions were extracted and amplified with the hepatitis B virus nucleic acid assay kit (PCR-fluorescent probe method) of Sun Yat-Sen University Daan Gene Co., Ltd.
[0036] Control group: negative seru...
Embodiment 3
[0043] Embodiment 3 storage stability test
[0044] 1. Instruments, reagents and samples
[0045] Main instruments: dry constant temperature metal bath, FLEX extractor, biological safety cabinet, pipette, Hongshi real-time fluorescence quantitative PCR instrument, etc.
[0046] Main Reagent: Hepatitis B Virus Nucleic Acid Detection Kit (PCR-fluorescent probe method) from Sun Yat-Sen University Daan Gene Co., Ltd.
[0047] Sample: fresh HCV pseudovirus sample
[0048] 2. Experimental plan
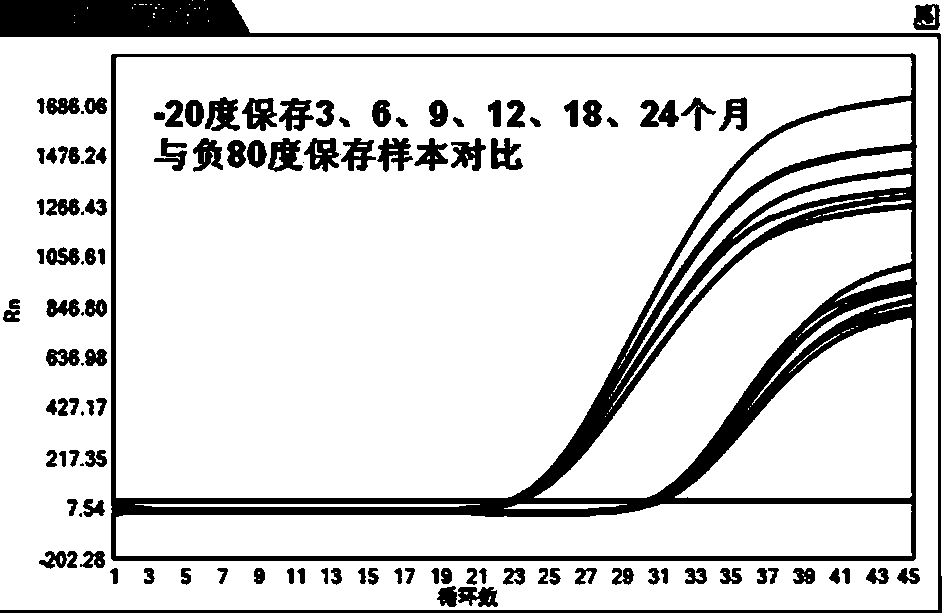
[0049] Dilute the HBV positive samples 100 times and 1000 times with the hepatitis B virus (HBV DNA) serum sample storage diluent prepared in Example 1, and use them as medium and low concentration test samples, which are marked as P1 and P2 respectively. Place them in -20±5°C environment, 2-8°C environment, room temperature (20-25°C) environment and 37°C environment respectively. The design of this experiment is as follows: mark the diluted samples and place them in the In the specified...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com