Quality control product suitable for quantitative detection of tumor marker Pro-GRP
A tumor marker, quantitative detection technology, applied in the field of quality control products, can solve the problems of inability to meet the normal use of the laboratory, poor stability and uniformity, and inability to be suitable for detection, and achieve stable activity, long stability time, and high accuracy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Preparation of Quality Control Products Suitable for Quantitative Detection of Tumor Marker Pro-GRP
[0016] Concrete preparation steps are:
[0017] The first step is to select the hormone-free serum suitable for the tumor marker Pro-GRP (purchased hormone-free human serum, the background value of Pro-GRP in the serum is ≤100pg / ml), and store it at 2-8°C after thawing;
[0018] In the second step, add 1-5g of ADP, 1-5ml of Proclin300, 1-5g of gentamicin sulfate, and 50-100g of trehalose to each liter of hormone-free serum in the first step, and stir well , to obtain a clear and translucent matrix solution, which should be stored at 2-8°C;
[0019] In the third step, take five equal volumes of matrix solution (500ml each), add 0.31μl, 1.43μl, 2.78μl, 3.85μl, 5.56μl of the same titer of tumor marker Pro-GRP antigen to obtain 1-80pg / mL, 80-200pg / mL, 200-800pg / mL, 800-1500pg / mL, 1500-5000pg / mL five groups of primary liquid tumor marker Pro-GRP quality control ...
Embodiment 2
[0025] Example 2 Performance test of the tumor marker Pro-GRP quality control product prepared by the present invention
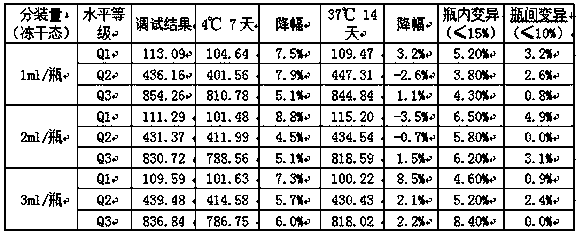
[0026] The stability and uniformity of the freeze-dried tumor marker Pro-GRP quality control products with filling volumes of 1mL / bottle, 2mL / bottle, and 3mL / bottle were tested for stability and uniformity, and the specific results are as follows:
[0027]
[0028] It can be seen from the above table that the lyophilized tumor marker Pro-GRP quality control product can be stored at 2-8°C for 7 days after reconstitution after opening the bottle (decrease ≤15%), the variation within the bottle is ≤15%, and the variation between the bottles is ≤10 %, effectively improving the stability and uniformity of Pro-GRP.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com