Preparation method of anti-Mullerian hormone quality control substance
A quality and hormone technology, applied in the field of preparation of anti-Mullerian hormone quality control products, can solve the problems of affecting detection, plasma matrix precipitation, etc., and achieve the effect of high consistency, good stability and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Preparation of Anti-Müllerian Hormone Quality Control Products
[0026] The first step is to use commercial hormone-free serum to measure HBsAg, hepatitis C antibody, syphilis antibody, and HIV antibody in the serum respectively;
[0027] In the second step, select hormone-free serum that is negative for HBsAg, hepatitis C antibody, syphilis antibody, and HIV antibody, and then add 0.2% sodium azide, 0.2% Proclin300, 0.2% gentamicin sulfate, and 0.2% thimerosal , 5% trehalose, and mix well;
[0028] The third step is to measure the background value of anti-Müllerian hormone and store it temporarily at 2-8°C;
[0029] In the fourth step, according to the background value determined in the third step, anti-Müllerian hormone-positive substances are added, and the concentrations are 1.0ng / ml, 5.0ng / ml, and 15.0ng / ml respectively;
[0030] The fifth step is to store the substance obtained in the fourth step at a temperature of 2-8°C for 3-14 days, so that the com...
Embodiment 2
[0031] The performance measurement of the quality control product prepared in embodiment 2 embodiment 1
[0032] 1. Stability
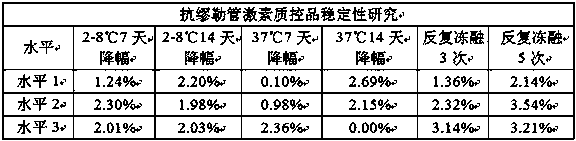
[0033] Three-level (1.0ng / ml, 5.0ng / ml, 15.0ng / ml) quality control products were tested in turn by storing them at 2-8°C after reconstitution, accelerating at 37°C in a freeze-dried state, and testing the number of freeze-thaw cycles after reconstitution. The test data are shown in Table 1 below:
[0034] Table 1
[0035]
[0036] From the data in the table, it can be seen that the anti-Müllerian hormone quality control product was opened and reconstituted, stored at 2-8°C for 14 days, heat-accelerated at 37°C for 14 days, and after repeated freezing and thawing for 5 times, the decrease was less than 5%. The stability meets the requirements.
[0037] 2. Check the consistency between platforms
[0038] Three-level (1.0ng / ml, 5.0ng / ml, 15.0ng / ml) quality control substances were tested on mainstream platforms (Roche, Beckman) respectively. The te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com