Gentamicin sulfate prepared through enhanced microbial fermentation, and application method of gentamicin sulfate
A technology of gentamicin and antibacterial drugs, which is applied in the field of directional high-yield gentamicin C2 engineering bacteria and antibiotic manufacturing, which can solve the problems of pollution, interference, low yield, etc., meet the requirements of industrialization and shorten the production cycle , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
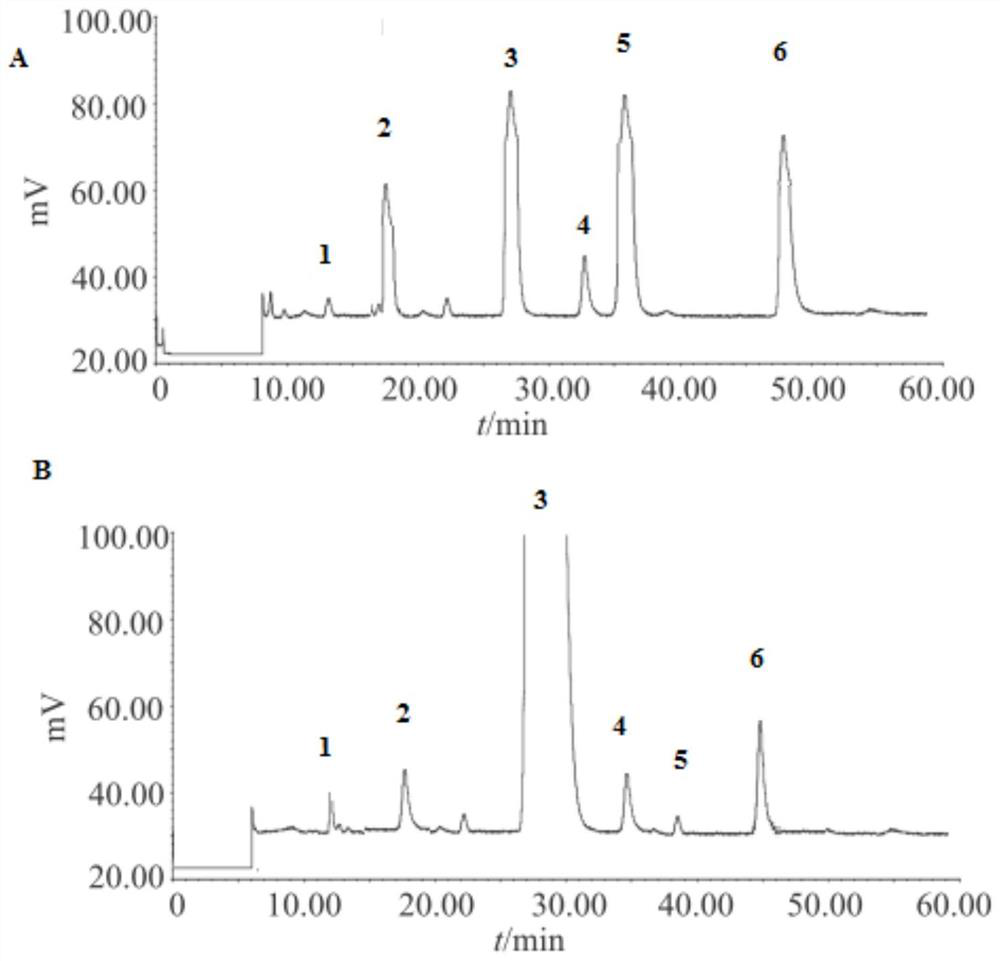
Image
Examples
Embodiment 1
[0016] Example 1 Atmospheric room temperature plasma mutagenesis technology screening
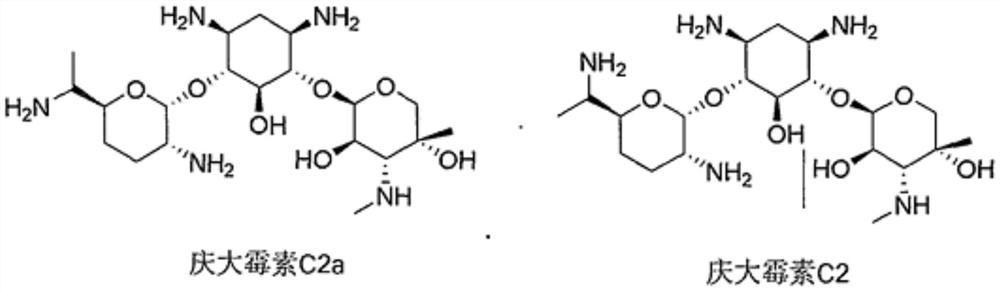
[0017] 1) With the gentamicin production strain Micromonospora rubrum SD2018 in the applicant's strain bank as the starting strain, use a sterilized 1ml straw to draw 0.1ml and inoculate it on the sterilized slant medium for slanting Spore culture, culture temperature is 35~37 ℃, stand still, culture time is 4 days, obtain the slant spore of SD2020 strain;
[0018] 2) Take 1.0cm 2 Step 1) Add the slanted spores prepared into a sterilized Erlenmeyer flask containing 5ml of saline with a mass concentration of 0.9%, and shake for 20 minutes to obtain a spore suspension;
[0019] 3) Take 500 μl of the spore suspension prepared in step 2), add 250 μl of 20% lithium chloride aqueous solution and 250 μl of 0.9% normal saline, shake and mix for 5 minutes to obtain spores containing 5% lithium chloride Suspension; after shaking and mixing, draw 20 μl of spore suspension containing 5% lithium chlor...
Embodiment 2
[0021] The efficient fermentation of embodiment 2 gentamicin C2
[0022]The Plackett-Burman design method is a nearly saturated two-level experimental design method developed in the middle and late 20th century. Based on the principle of incomplete balance blocks, it can estimate the main effect of factors with the least number of trials, and quickly and effectively screen out the most important factors from many factors for further research. This method is simple in data processing. Compared with the currently commonly used partial factor test and random balance test, this method is the most efficient and accurate in screening important factors. Therefore, it has been widely used in the optimization of microbial culture conditions and achieved good results. In the embodiment, it is planned to investigate and evaluate many factors in the fermentation medium formula of gentamycin C2 through the Plackett-Burman experimental design of DesignExpert7.0, and screen out several impor...
Embodiment 3
[0036] The refining of embodiment 3 gentamicin C2
[0037] Utilize the above-mentioned fermentation method to carry out expanded cultivation, add 10% tap water to dilute the fermented liquid after expanded fermentation, add hydrochloric acid or sulfuric acid, acidify to pH1.0-3.0, fully stir 1-4 hour; pH5.0-7.0, put in 732NH 4+ Resin static adsorption for 6-8 hours. The amount of resin input is calculated at about 50,000 u / mL. Collect the adsorbed saturated resin and rinse it with tap water (until there is no floating mycelium). Pack the saturated resin into a column, pickle the saturated resin with 0.5M HCl solution twice the volume of the saturated resin, then wash it with ion-free water until neutral, and then use 0.01% ammonia water for alkaline washing. When the effluent reaches pH 9.0 or above, Connect in series to an equal volume of 711 resin column, use 4% ammonia water for elution, the amount of ammonia water is 8-10 times the volume of saturated resin, the elution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com