Combined porcine transmissible gastroenteritis and porcine epizootic diarrhea living vaccine cryoprotectant and combined living vaccine
A technology for porcine epidemic diarrhea and freeze-dried protective agent, which is used in vaccines, freeze-dried transportation, veterinary vaccines, etc., can solve the problems of slow dissolving speed of freeze-dried products, depressions on the surface, loss of titer, etc., and reduce the drug price. loss, smooth surface, improved protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
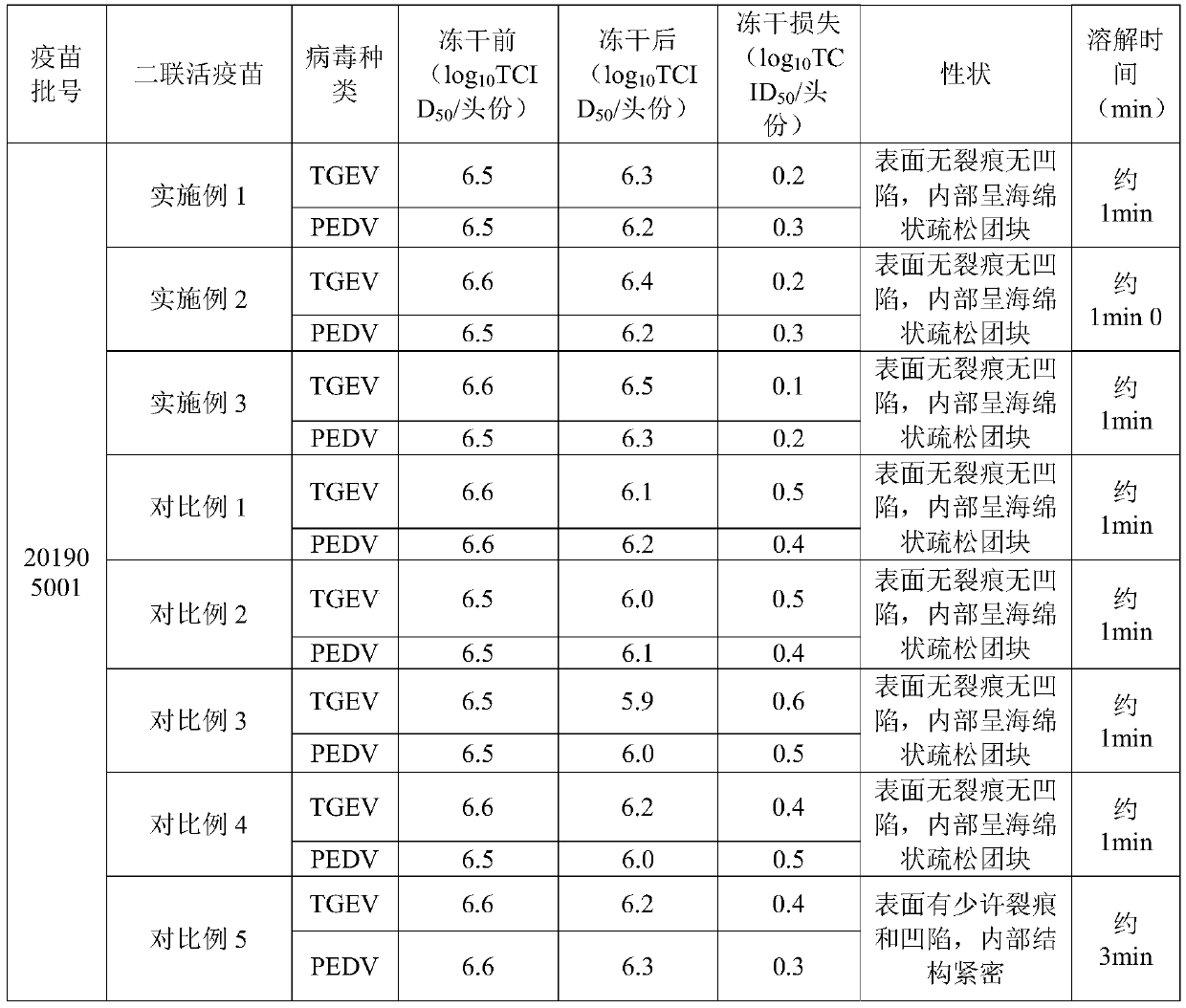
[0017] A freeze-dried protective agent for porcine transmissible gastroenteritis and porcine epidemic diarrhea dual live vaccine, which is composed of the following components in mass and volume percentages: 20% sucrose, 6% gelatin, 2% tryptone, 1% yeast extract powder, Chondroitin sulfate 0.1% and inositol 1%, the balance is water for injection.
[0018] A dual live vaccine, comprising a mixed virus liquid containing porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus and the above-mentioned porcine transmissible gastroenteritis, porcine epidemic diarrhea dual live vaccine lyoprotectant, wherein, The volume ratio of the mixed virus solution to the lyoprotectant is 4:1.
[0019] The preparation process of the above-mentioned dual live vaccine is as follows: (1) mixing and dissolving each component in the lyoprotectant, and then performing sterilization treatment; (2) mixing porcine transmissible gastroenteritis virus and porcine epidemic diarrhea vi...
Embodiment 2
[0021] A freeze-dried protective agent for porcine transmissible gastroenteritis and porcine epidemic diarrhea dual live vaccine, which consists of the following components in mass and volume percentages: 28% sucrose, 10% gelatin, 6% tryptone, 3% yeast extract powder, Chondroitin sulfate 2% and inositol 5%, the balance is water for injection.
[0022] A dual live vaccine, comprising a mixed virus liquid containing porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus and the above-mentioned porcine transmissible gastroenteritis, porcine epidemic diarrhea dual live vaccine lyoprotectant, wherein, The volume ratio of the mixed virus solution to the lyoprotectant is 8:1.
[0023] The preparation process of the above-mentioned dual live vaccine is as follows: (1) mixing and dissolving each component in the lyoprotectant, and then performing sterilization treatment; (2) mixing porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus ...
Embodiment 3
[0025] A freeze-dried protective agent for porcine transmissible gastroenteritis and porcine epidemic diarrhea dual live vaccine, which is composed of the following components in mass and volume percentages: 24% sucrose, 8% gelatin, 4% tryptone, 2% yeast extract powder, Chondroitin sulfate 1.5% and inositol 3%, the balance is water for injection.
[0026] A dual live vaccine, comprising a mixed virus liquid containing porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus and the above-mentioned porcine transmissible gastroenteritis, porcine epidemic diarrhea dual live vaccine lyoprotectant, wherein, The volume ratio of the mixed virus solution to the lyoprotectant is 7:1.
[0027] The preparation process of the above-mentioned dual live vaccine is as follows: (1) mixing and dissolving each component in the lyoprotectant, and then performing sterilization treatment; (2) mixing porcine transmissible gastroenteritis virus and porcine epidemic diarrhea vi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com