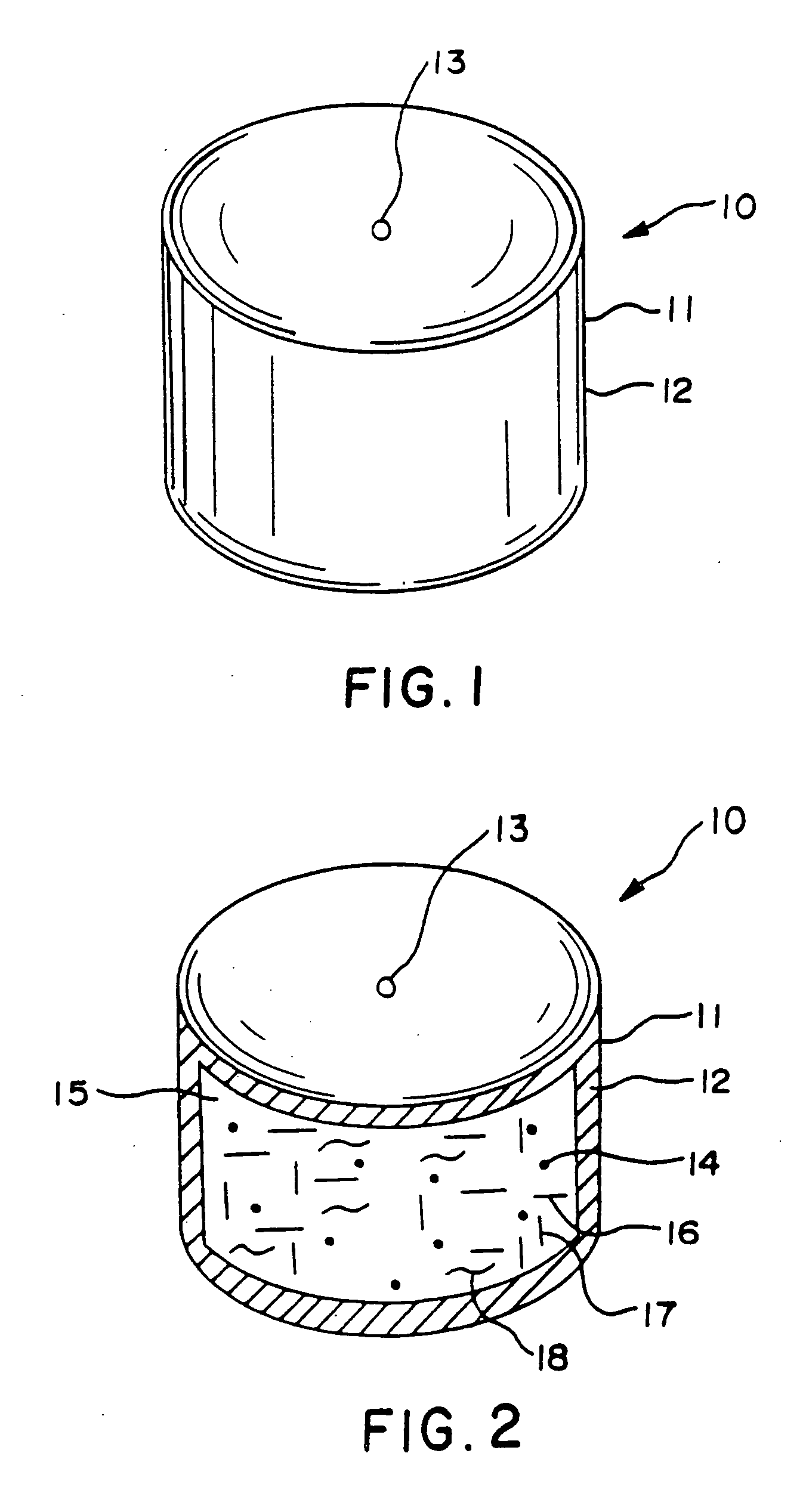
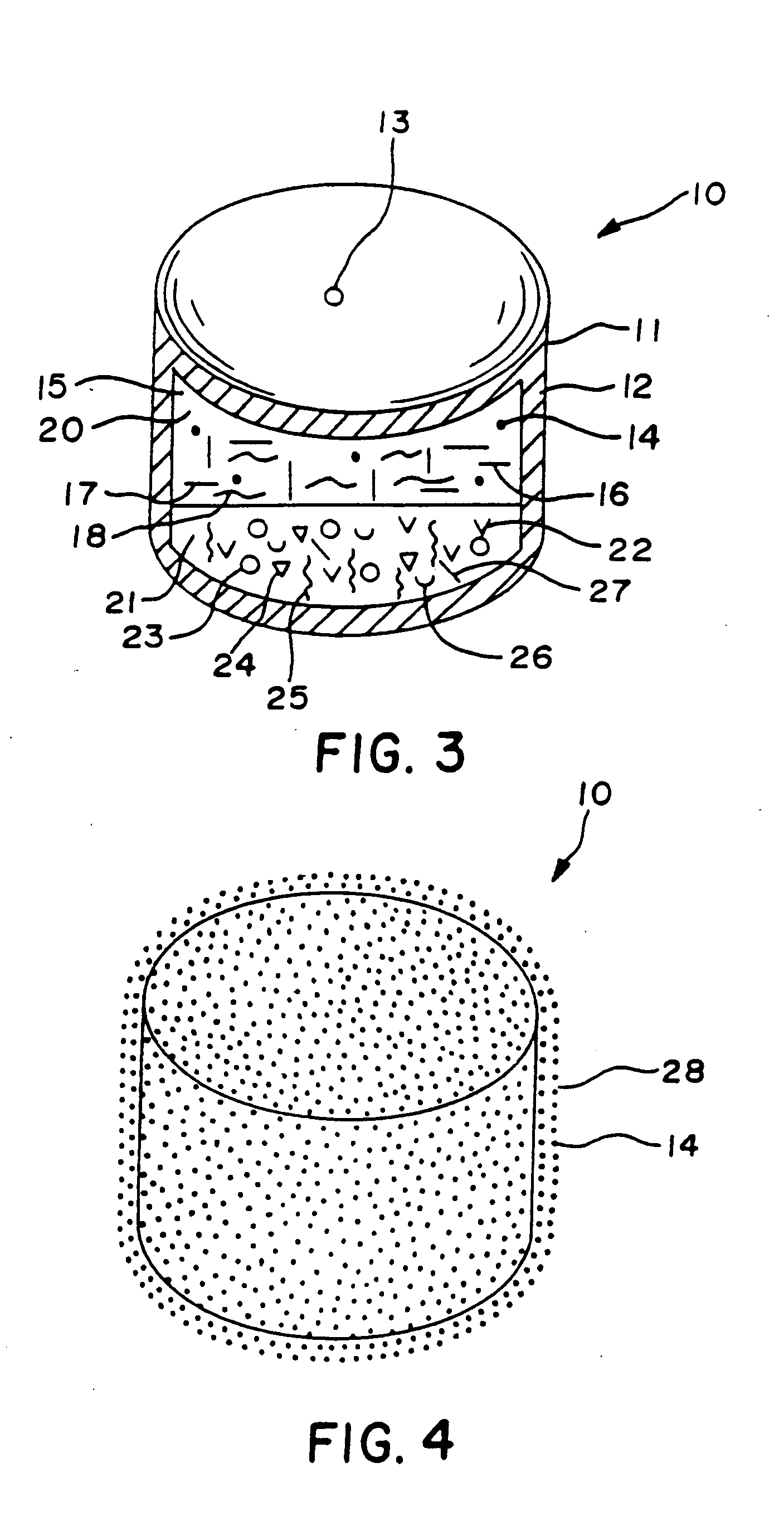
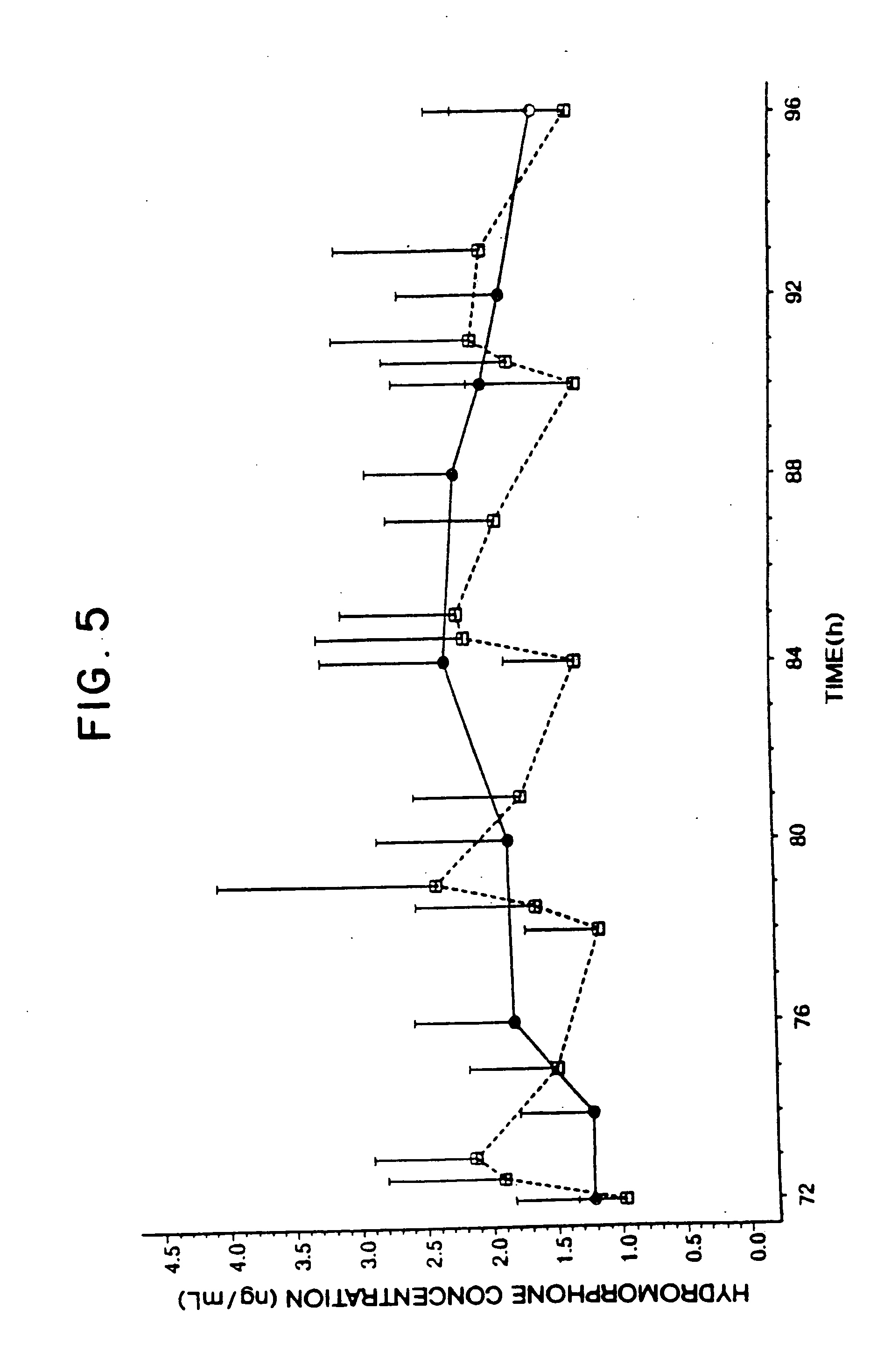
Hydromorphone therapy
a technology of hydromorphone and hydromorphone, which is applied in the field of hydromorphone therapy, can solve the problems of changing the release rate of hydromorphone from polyalkylene oxide, conventional dosage forms, and a lack of pharmaceutical and medical arts, so as to suppress anxiety and apprehension, relieve pain, and lessen pain for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0053] A novel therapeutic composition comprising hydromorphone, wherein the hydromorphone is a member selected from the group consisting of hydromorphone pharmaceutically acceptable base and hydromorphone pharmaceutically acceptable salt, is prepared as follows: First, 175 g of hydromorphone hydrochloride, 647.5 g of poly(ethylene oxide) possessing a 200,000 molecular weight, and 43.75 g of poly(vinyl pyrrolidone) having an average-molecular weight of 40,000 are added to planetary mixing bowl and the ingredients dry mixed for ten minutes. Then, 331 g of denatured anhydrous alcohol is slowly added to the blended ingredients, with continuous blending for approximately ten minutes. Next, the freshly prepared wet granulation is passed through a 20-mesh screen, allowed to dry at 25.degree. C. for about 20 hours, and then passed through a 16-mesh screen. Next, the granulation is transferred to the planetary mixer and lubricated with 8.75 g of magnesium stearate to produce a therapeutic h...
example 2
[0054] The therapeutic compositions manufactured by following the above example provide compositions comprising 1 to 500 mg of a member selected from the group consisting of hydromorphone, hydromorphone base, hydromorphone salt and hydromorphone derivative; at least one polymeric carrier for the hydromorphone selected from 20 to 375 mg of poly(alkylene oxide) comprising a 50,000 to 750,000 molecular weight represented by poly(methylene oxide), poly(ethylene oxide), poly(propylene oxide), poly(isopropylene oxide) and poly(butylene oxide)., or a polymeric carrier for the hydromorphone consisting of 20 to 375 mg of carboxymethylcellulose having a 10,000 to 175,000 molecular weight represented by a member selected from the group consisting of alkali carboxymethylcellulose, sodium carboxymethylcellulose and postassium carboxymethylcellulose; 0.01 to 25 mg of poly(vinyl) polymer possessing a 5,000 to 350,000 molecular weight represented by poly(vinyl pyrrolidone), copolymer of poly(vinyl ...
example 3
[0055] The therapeutic composition provided by the invention can be dry compressed into an orally administrable dosage form. For example, a mixture of dry-powder ingredients comprising a hydromorphone pharmaceutically acceptable base or a hydromorphone pharmaceutically acceptable salt as represented by: hydrochloride, hydrobromide, sulfate, bisulfate, acetate, valerate, oxalate, oleate, laureate, borate, benzoate, lactate, phosphate, tosylate, citrate, maleate, fumarate, succinate, tartrate and napsylate; a tablet excipient represented by 0 to 200 mg of microcrystalline cellulose; 20 to 375 mg of sodium carboxymethylcellulose of 10,000 to 175,000 molecular weight; 0.01 to 25 mg of a binder agent represented by poly(vinyl pyrrolidone) of 5,000 to 350,000 molecular weight, a hydroxypropylmethylcellulose of 9,200 to 75,000 molecular weight, and gelatin; and 0 to 10 mg of a lubricant, such as stearic acid, calcium stearate or magnesium stearate; are dried, sieved and mixed with other op...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wt % | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com