Method for establishing non-alcoholic fatty liver disease combined with viral hepatitis mouse model
A technology for fatty liver disease and viral hepatitis, which is applied in the field of biomedical research to achieve the effects of low cost, complete virus infection and replication cycle, and prevention of deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 1. High-fat diet induction treatment
[0062] 1A) Select 125 SPF female C3H / HeN mice aged 6-7 weeks and feed them with high-fat diet (referred to as high-fat diet group mice). The high-fat diet was purchased from Nantong Trophy Feed Technology Co., Ltd. , product number TP23520, each kilogram of feed contains 258g casein, 162g dextrin, 89g sucrose, 32g soybean oil, 317g lard, 65g cellulose, 58g minerals, 13g vitamins, 4gL-cystine, 3g choline bitartrate and 0.069g food antioxidants;
[0063] In the present invention, the experimental mice are selected as docile female mice, which is not only convenient for raising but also beneficial for the smooth progress of the test treatment.
[0064] 1B) Take another 15 SPF grade female C3H / HeN mice aged 6-7 weeks and feed them with normal diet (abbreviated as normal control group mice);
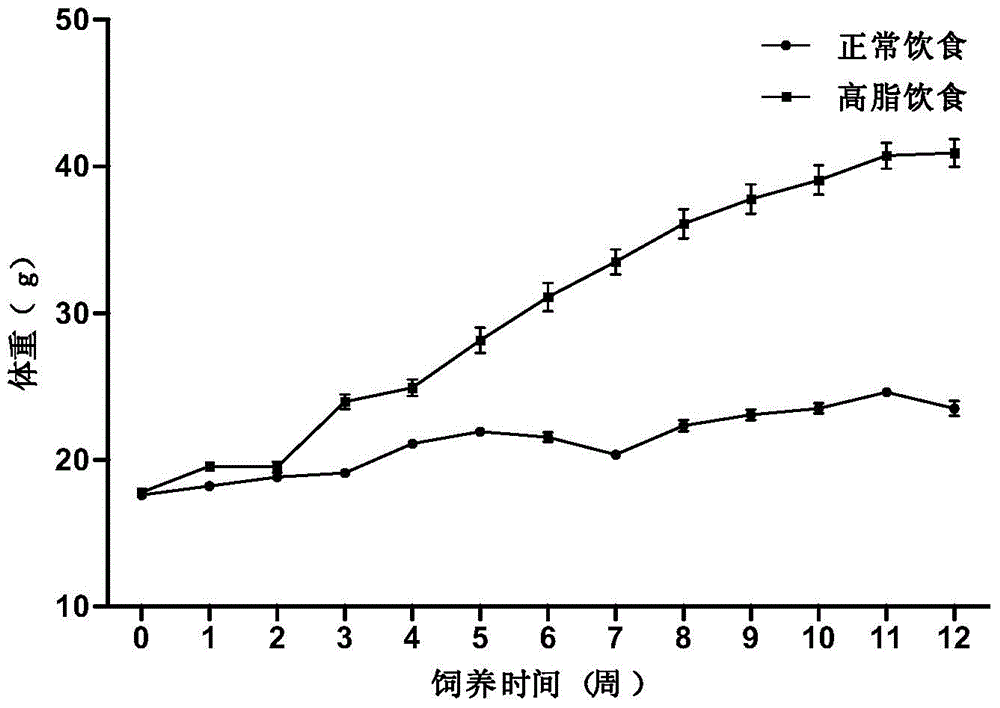
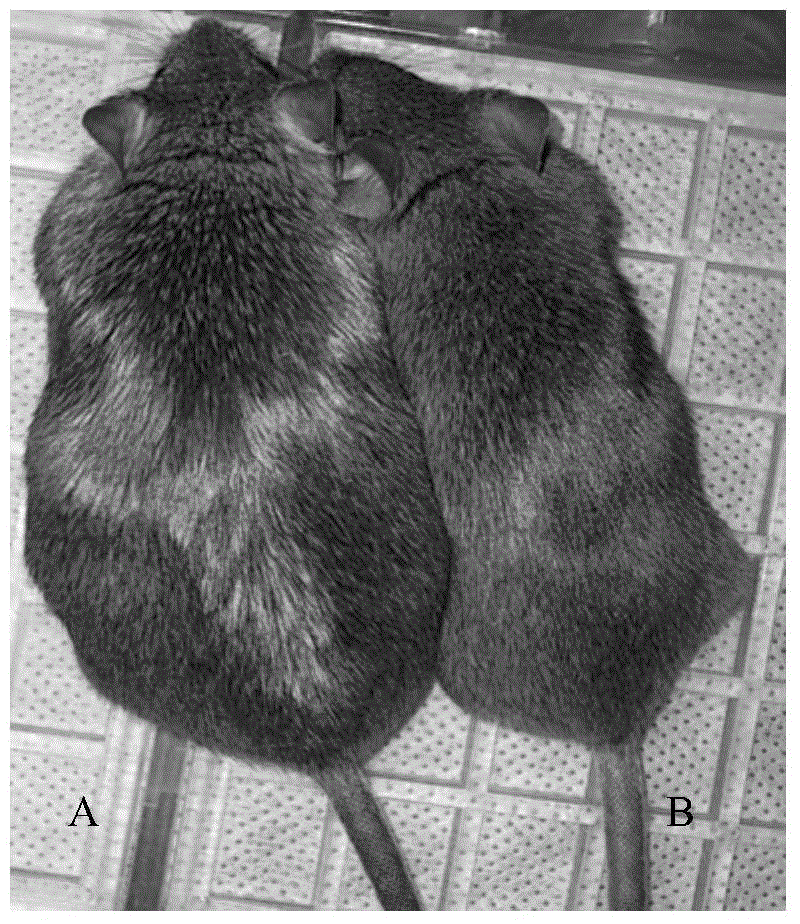
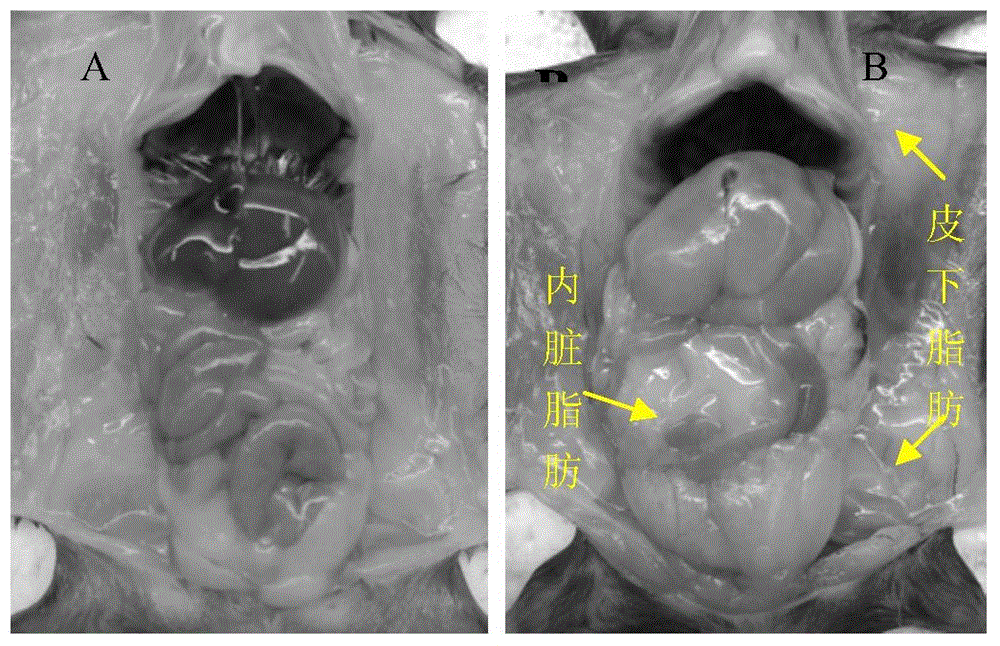
[0065] 1C) The weights of the two groups of mice were weighed every week, and the general conditions of the mice were observed. After 12 weeks ...
Embodiment 2
[0094] Except step 2B) set the titer to 1.0×10 6 PFU / ml of murine hepatitis type 3 virus was diluted to 5 PFU / 100 μl with sterile PBS buffer; in step 2C), each mouse was injected with 5 PFU of murine hepatitis type 3 virus, and the others were the same as in Example 1.
[0095] 20 infected mice were randomly selected to observe the state and survival rate of the mice. The mice showed symptoms such as listlessness, erect hair and dullness, curled up and trembling, and sluggishness within 5-15 days of virus infection; 4 mice were infected Death occurred on the 6th-13th day, and the survival rate was 80%; after 15 days of infection, the mice did not die again;
[0096] After the mice were infected with murine hepatitis type 3 virus, the peripheral blood of the mice was collected on the 4th day, 8th day, 12th day, 16th day, 20th day, and 30th day to detect transaminase, and the liver tissue was collected for hematoxylin-eosin staining. The peripheral blood and liver tissue of mic...
Embodiment 3
[0101] Except step 2B) set the titer to 1.0×10 6 The pFu / ml murine hepatitis type 3 virus was diluted to 15 PFU / 100 μl with sterile PBS buffer; step 2C) except that 15 PFU of murine hepatitis type 3 virus was injected into each mouse, the rest were the same as in Example 1.
[0102] 20 infected mice were randomly selected to observe the state and survival rate of the mice. The mice had symptoms such as listlessness, erect hair and dullness, curled up and trembling, and slow movement within 3-15 days of virus infection; 7 mice were infected Death occurred on the 3rd to 12th day, and the survival rate was 65%. After 15 days of infection, the symptoms of the mice were gradually relieved, and no death occurred again;
[0103] After the mice were infected with murine hepatitis type 3 virus, the peripheral blood of the mice was collected on the 4th day, 8th day, 12th day, 16th day, 20th day, and 30th day to detect transaminase, and the liver tissue was collected for hematoxylin-eosi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com