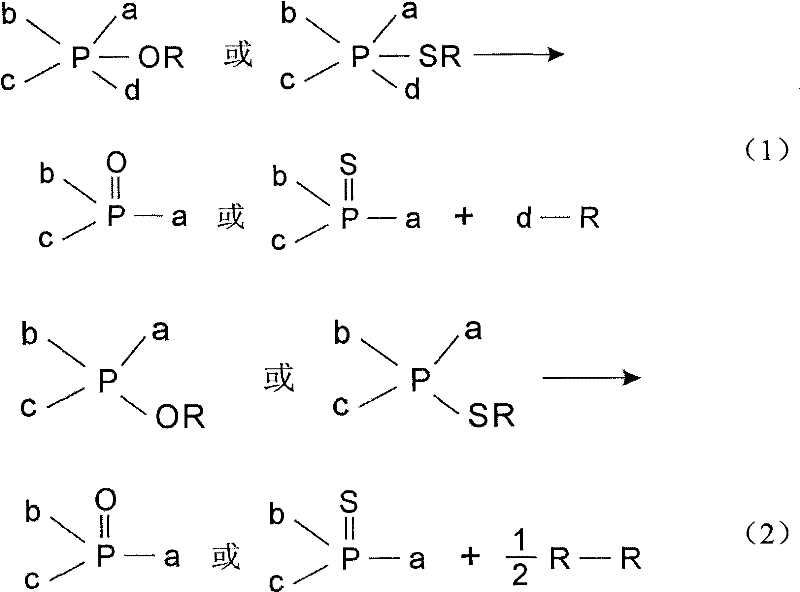
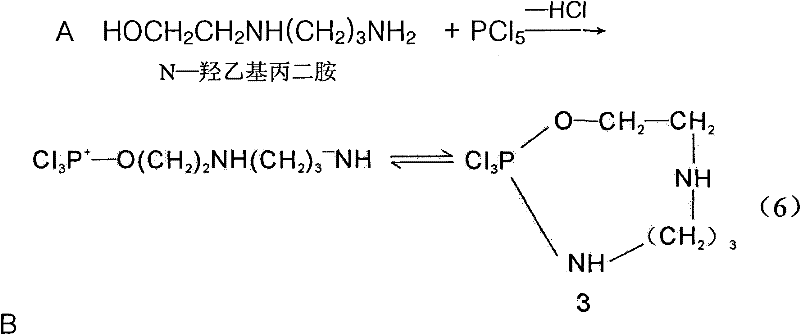
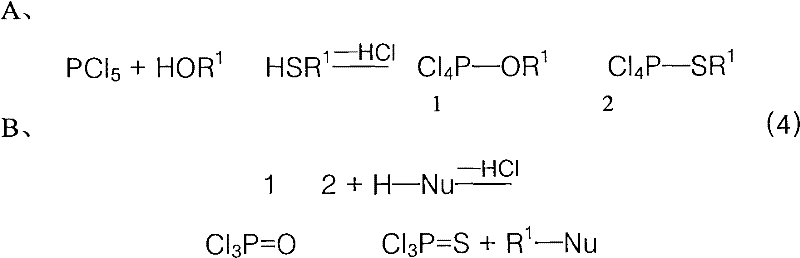Application of LJ reaction in mitsunobu reaction
A reaction and new light technology, applied in compounds of group 5/15 elements of the periodic table, phosphorus halides/oxyhalides, organic chemistry, etc., can solve the problems of increasing the emission of "three wastes", production costs, and removal difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 Molecular ratio: N-hydroxyethylpropylenediamine: PCl 5 =1:1~2
[0046] Under the condition of isolating air and water, 30 grams of PCl 5 with 70 g CCl 4 Put it into the reaction bottle, start stirring, control the temperature at 30-60°C, add N-hydroxyethylpropylenediamine dropwise at the slowest speed, after the drop is finished, keep it warm for about 20 hours, and then azeotropically distill it under high vacuum Steam out phosphorus oxychloride as much as possible, add 50 grams of dichloroethane, and then wash excess PCl with 5% NaOH alkaline water 5 , separate layers, separate the water layer, distill the solvent of the organic layer, crystallize, filter, and dry to obtain homopiperazine with a content of 95% and a yield of 90%.
Embodiment 2
[0047] The synthesis of embodiment 2 benzoic acid (2-phenyl-2-butyl) ester
[0048] At 0°C under an argon atmosphere, phosphorus pentachloride (1.5 mol) in CCl 4Solution (30%) is dropped in reaction flask, drips the mixed n-hexane solution (40%) of 2-phenyl-2-butanol (0.6mol) and benzoic acid (0.6mol), insulation reaction 18~26 hours, then , under high vacuum as far as possible azeotropic steaming of phosphorus oxychloride, and then wash off excess phosphorus pentachloride with 5% NaOH alkaline water, layering, distillation, crystallization, filtration, drying to obtain the product, the content is 90%, the yield is 85% %.
Embodiment 3
[0049] The synthesis of embodiment 3 acetic acid 2-phenyl-2-benzyloxycarbonylaminopropylthioester
[0050] Under the condition of isolating air and water, and stirring at -10°C, the PCl 5 (10mol) of CCl 4 The solution (30%) is put into the reaction kettle, and the dry solution containing 2-phenyl-2-benzyloxycarbonylaminopropylthioester (5mol) and thioacetic acid (5mol) is added dropwise at the slowest speed, and continued at room temperature Stir and react for 3 to 6 hours, then try to azeotropically distill phosphorus oxychloride under high vacuum, and recycle it, then wash off excess phosphorus pentachloride with 5% NaOH alkaline water, separate layers, distill, crystallize, filter, The product was obtained by drying with a content of 92% and a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com