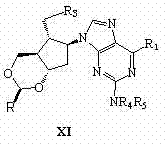
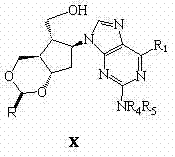
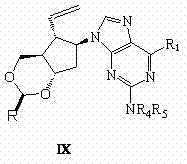
Synthetic method of entecavir
A technology of entecavir and a synthesis method, which is applied in the field of synthesis of pharmaceutical intermediates and entecavir, can solve the problems such as difficulty in obtaining raw materials, low yield, complicated reaction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment one: the preparation of compound III-1
[0050]
[0051] Dissolve (+)-Corey diol (17.2g, 1eq) in 100mL tetrahydrofuran, add 4-methylbenzenesulfonic acid (760mg, 0.04eq), then slowly add acetaldehyde diethyl acetal (23.6g, 2eq). After stirring for 15 minutes, heat to reflux and continue stirring for 4 hours. Remove from heat and cool to room temperature. Add 20 ml of saturated sodium bicarbonate and extract with ethyl acetate. The organic phase was concentrated to 50ml, and 150ml of petroleum ether was slowly added under stirring, and a solid was precipitated to obtain compound III-1 (16.8g, 85% yield). 1 H NMR (CDCl 3 , 400 MHz) δ 1.36 (d, J=5.2 Hz ,3H, CH 3 ), 1.64-1.73 (m, 1H), 1.83-1.90 (m, 1H), 2.30-2.44 (m, 2H), 2.62-2.73 (m, 2H), 3.38-3.45 (m, 1H), 3.58 (t , J=10.8 Hz, 1H), 4.28 (dd, J=4.4Hz, 10.8 Hz, 1H), 4.70(q, J=4.8 Hz, 1H), 4.88-4.92 (m, 1H); 13 C NMR (CDCl 3 , 100 MHz) δ 20.6,32.4, 36.6, 36.9, 45.3, 70.4, 79.7, 80.5, 99.7,176.0; Ms (+C,...
Embodiment 2
[0052] Embodiment two: the preparation of compound III-2
[0053]
[0054] (+)-Corey diol (17.2 g, 1 eq) was dissolved in 100 mL of toluene, 4-methylbenzenesulfonic acid (760 mg, 0.04 eq) was added, followed by the slow addition of benzaldehyde (21.2 g, 2 eq) at room temperature. Then it was heated to reflux temperature, and the water was separated by reflux through a water separator, and stirred for 6 hours. Remove from heat and cool to room temperature. Add 20 ml of saturated sodium bicarbonate and extract with ethyl acetate. The organic phase was concentrated to 50ml, and 150ml of petroleum ether was slowly added under stirring, and a solid was precipitated to obtain compound III-1 (18.5g, 71% yield). 1 H NMR (CDCl 3 , 400 MHz) δ1.72-1.75 (m, 1H), 2.14-2.19 (m, 1H), 2.39-2.53 (m, 2H), 2.81-2.88 (m, 1H), 3.36-3.38 (m, 1H) , 4.13 (d, J=14.8 Hz, 1H), 4.25 (d, J=14.8 Hz, 1H), 4.48(dd, J=3.2 Hz, J=4.4 Hz, 1H), 5.15-5.18 (m, 1H) , 5.48 (s, 1H), 7.36-7.41 (m, ArH, 3H), 7.4...
Embodiment 3
[0055] Embodiment three: the preparation of compound IV-1
[0056]
[0057] Compound III (19.8g, 1eq) was dissolved in 400mL of anhydrous tetrahydrofuran at 0 o Add Lithium Aluminum Hydride (3.8g, 1eq) in batches at 0°C, keeping the internal temperature below 10 o C, the addition was completed within 30 min, and after the addition was completed, it was slowly raised to room temperature and continued to stir for 3 hours. Then, the reaction solution was cooled to 0 o C, slowly drop 30% sodium hydroxide solution to quench the reaction. Filter and wash the filter residue with ethyl acetate. The crude compound IV-1 from the concentrated filtrate (19 g, 95% yield, can be directly used in the next step). 1 H NMR (CDCl 3 , 400 MHz) δ1.36 (d, J=4.8 Hz , 3H, CH 3 ), 1.45-1.61 (m, 3H), 1.70-1.75 (m, 1H), 1.84-1.90 (m, 1H), 2.51-2.58 (m, 1H), 3.29-3.34 (m, 1H), 3.50 (t , J=10.8 Hz, 1H), 3.55-3.61 (m, 1H), 3.77-3.80 (m, 1H), 4.19 (dd, J=4Hz, 10.4 Hz, 1H), 4.30 (q, J=6.8 Hz, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com