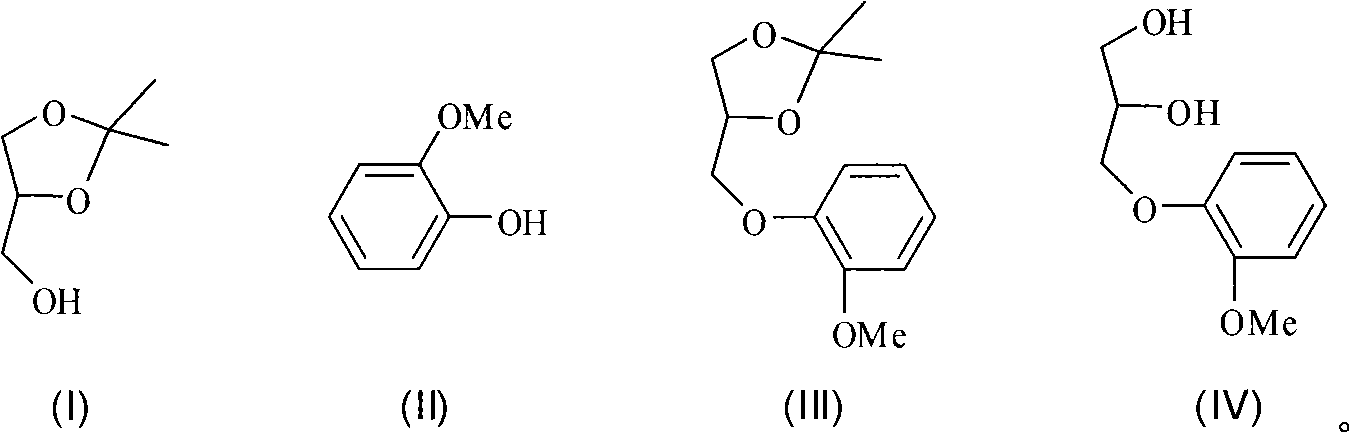
New synthetic method of guaiacol glycerin ether
A technology of guaiacol glycerin and synthesis method, which is applied in the field of medicine and chemical industry, can solve the problems of adverse effects on the environment and human body, high boiling point of solvent DMF, and numerous steps, and achieve the effects of simplified operation, simple post-processing, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
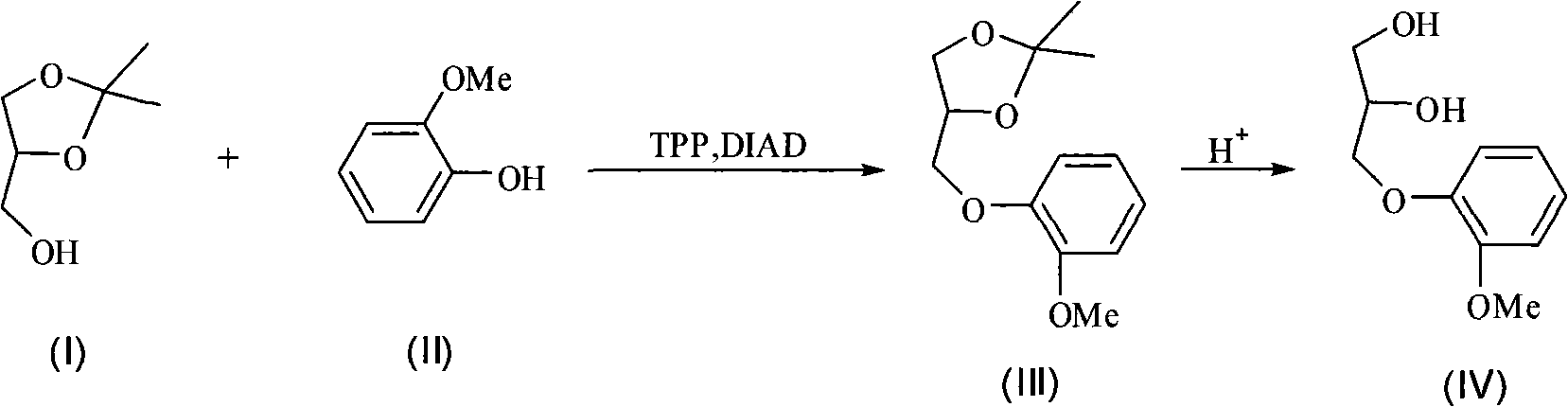
[0031] Add 1.32g (0.010mol) isopropylidene glycerol, 1.24g (0.010mol) guaiacol, 2.62g (0.010mol) triphenylphosphine, 10mL tetrahydrofuran into a 50mL three-necked flask, slowly add 2.0 mL (0.010mol) of diisopropyl azodicarboxylate, after the dropwise addition, the temperature was raised to 60°C. After reacting for 12 hours, it was washed with 80 mL of 5% sodium hydroxide solution for 4 times, and with 60 mL of saturated saline for 3 times. , 40 mL of clear water and washed twice, then dried and concentrated to obtain the crude intermediate product. Add 20 mL of 10% hydrochloric acid to the above crude product, and heat to 80° C. for 4 h. After cooling, add 25 mL of 10% sodium hydroxide solution to neutralize. Acetone was distilled off. Cool, filter, and extract the filtrate with dichloromethane. The extract was dried, concentrated, and recrystallized from toluene to obtain 0.75 g of guaiacol glycerol, with a yield of 38%, and a melting point of 78-79°C.
Embodiment 2
[0033] Add 1.32g (0.010mol) isopropylidene glycerol, 3.72g (0.030mol) guaiacol, 7.86g (0.030mol) triphenylphosphine, 30mL tetrahydrofuran into a 50mL three-necked flask, slowly add 6.0 mL (0.030mol) of diisopropyl azodicarboxylate, after the dropwise addition, the temperature was raised to 60°C. After reacting for 12 hours, it was washed with 80 mL of 20% sodium hydroxide solution for 4 times, and with 60 mL of saturated saline for 3 times. , 40 mL of clear water and washed twice, then dried and concentrated to obtain the crude intermediate product. Add 20 mL of 10% hydrochloric acid to the above crude product, and heat to 80° C. for 4 h. After cooling, add 25 mL of 10% sodium hydroxide solution to neutralize. Acetone was distilled off. Cool, filter, and extract the filtrate with dichloromethane. The extract was dried, concentrated, and recrystallized from toluene to obtain 1.58 g of guaiacol glyceryl ether, with a yield of 80%.
Embodiment 3
[0035] Add 1.32g (0.010mol) isopropylidene glycerol, 1.36g (0.011mol) guaiacol, 2.88g (0.011mol) triphenylphosphine, 20mL tetrahydrofuran into a 50mL three-necked flask, slowly add 2.2 mL (0.011mol) of diisopropyl azodicarboxylate, after the dropwise addition, the temperature was raised to 60°C, and after 12 hours of reaction, it was washed with 80 mL of 20% sodium hydroxide solution for 4 times, and 60 mL of saturated saline for 3 times. , 40 mL of clear water and washed twice, then dried and concentrated to obtain the crude intermediate product. Add 20 mL of 10% hydrochloric acid to the above crude product, and heat to 80° C. for 4 h. After cooling, add 25 mL of 10% sodium hydroxide solution to neutralize. Acetone was distilled off. Cool, filter, and extract the filtrate with dichloromethane. The extract was dried, concentrated, and recrystallized from toluene to obtain 1.54 g of guaiacol glyceryl ether, with a yield of 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com