Separation and purification method of ibrutinib intermediate
A technology of separation and purification, ibrutinib, applied in the direction of organic chemistry, etc., can solve the problems of high cost, cumbersome, difficult to remove triphenylphosphine, etc., to achieve the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
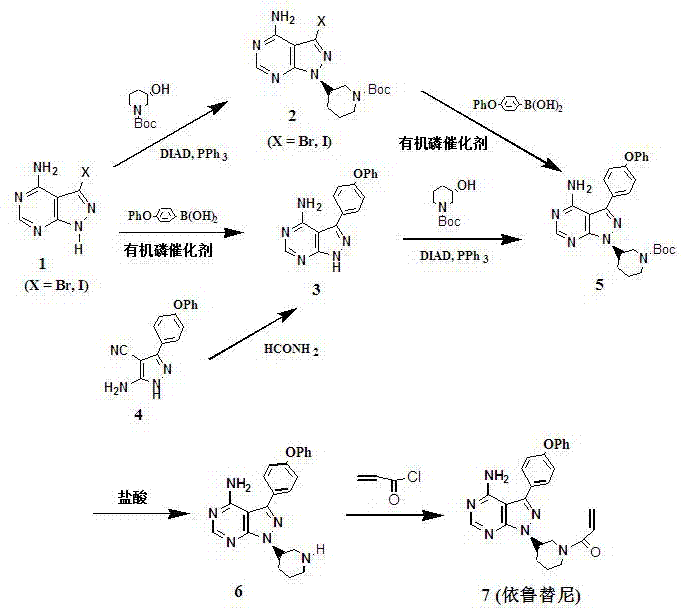
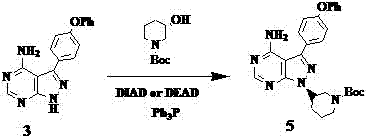
[0024] Under nitrogen protection, Ph 3 P (29.2g, 112mmol, 1.25eq), (3 S )-Hydroxy-1-tert-butoxycarbonylpiperidine (18.0g, 89.3mmol, 1.0eq) was dissolved in 150ml of tetrahydrofuran, cooled to 0°C, and the temperature was controlled not to exceed 5°C, and DIAD (25.3 g, 125mmol, 1.4eq) in tetrahydrofuran (40ml), after the addition was complete, the yellow solution continued to stir for 10min. Add 4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidine (28.4g, 93.8mmol, 1.05eq) in tetrahydrofuran at 0~5°C (150ml) solution, after addition, stirred at room temperature for 5h. Add 3.2ml of water, warm to 50-60°C for 30min, then add magnesium chloride (10.6g, 112mmol, 1.25eq), stir and warm to 50-60°C for 2.5h, cool to 0°C, filter, and wash with tetrahydrofuran (50ml×2 ), and the solvent was removed by rotary evaporation, the residual oil was slurried with ethyl acetate (300ml), added n-hexane (50ml), filtered, and the filter residue was washed with ethyl acetate (30ml×2), and th...
Embodiment 2
[0026] Under nitrogen protection, Ph 3 P (29.0g, 112mmol, 1.25eq), (3 S )-Hydroxy-1-tert-butoxycarbonylpiperidine (18.0g, 89.3mmol, 1.0eq) was dissolved in 150ml of tetrahydrofuran, cooled to 0°C, and the temperature was controlled not to exceed 5°C, and DIAD (25.1 g, 125mmol, 1.4eq) in tetrahydrofuran (40ml), after the addition was complete, the yellow solution continued to stir for 10min. Add 4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidine (28.4g, 93.9mmol, 1.05eq) in tetrahydrofuran at 0~5°C (150ml) solution, after addition, stirred at room temperature for 5h. Add 3.2ml of water, warm to 50-60°C for 30min, then add magnesium chloride (14.3g, 168mmol, 1.5eq), stir and warm to 50-60°C for 2.5h, cool to 0°C, filter, and wash with tetrahydrofuran (50ml×2 ), and the solvent was removed by rotary evaporation, the residual oil was slurried with ethyl acetate (300ml), added n-hexane (50ml), filtered, and the filter residue was washed with ethyl acetate (30ml×2), and the...
Embodiment 3
[0028] Under nitrogen protection, Ph 3 P (29.0g, 112mmol, 1.25eq), (3 S )-Hydroxy-1-tert-butoxycarbonylpiperidine (18.0g, 89.3mmol, 1.0eq) was dissolved in 150ml of tetrahydrofuran, cooled to 0°C, and the temperature was controlled not to exceed 5°C, and DIAD (25.1 g, 125mmol, 1.4eq) in tetrahydrofuran (40ml), after the addition was complete, the yellow solution continued to stir for 10min. Add 4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidine (28.2g, 93.9mmol, 1.05eq) in tetrahydrofuran at 0~5°C (150ml) solution, after addition, stirred at room temperature for 5h. Add 3.2ml of water, warm to 50-60°C for 30min, then add magnesium chloride (21.3g, 224mmol, 2.0eq), stir and warm to 50-60°C for 2.5h, cool to 0°C, filter, and wash with tetrahydrofuran (50ml×2 ), and the solvent was removed by rotary evaporation, the residual oil was slurried with ethyl acetate (300ml), added n-hexane (50ml), filtered, and the filter residue was washed with ethyl acetate (30ml×2), and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com