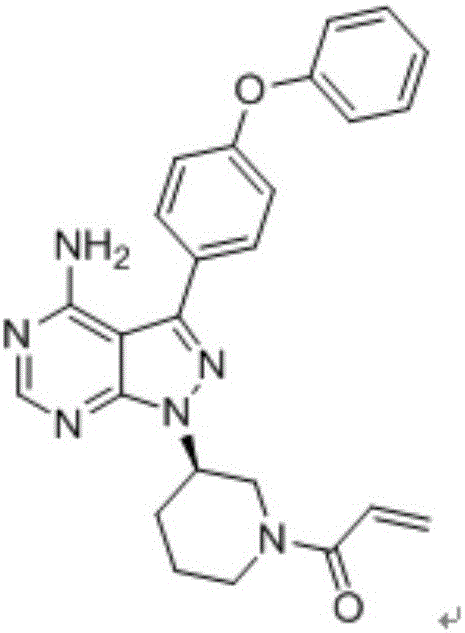
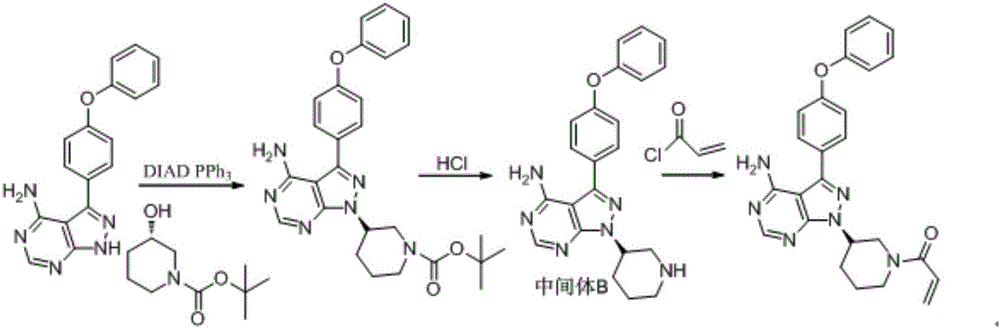
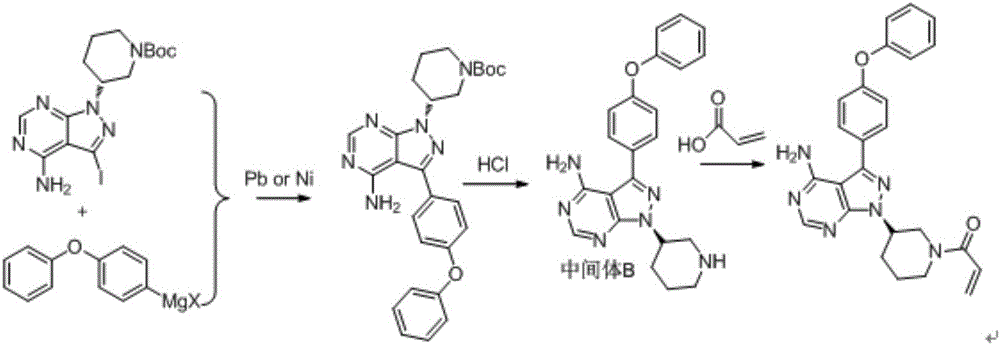
Synthesis and purification process of ibrutinib intermediates
A technology for ibrutinib and intermediates, applied in the field of ibrutinib intermediates and its recrystallization, can solve the problems of difficult control of the reaction process, high price, high cost, etc., achieve mild reaction conditions, simple experimental process, reduce cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Dissolve 10.0g (38.2mmol 1.0eq) of triphenylphosphine in 100ml of THF, cool at 0°C, add 7.67g (38.2mmol 1.0eq) of (s)-1-tert-butoxycarbonyl-3-hydroxypiperidine and 11.5g (38.2mmol 1.0eq) 3-(4-phenoxyphenyl)-1H-pyrazol[3,4-d]pyrimidin-4-amine, after stirring for 20 minutes, 7.67g (38.2mmol 1.0eq ) DIAD was added dropwise to the reaction liquid system, and kept at 0° C. for 20 hours. Then concentrate the feed solution to a viscous shape, add 380ml 0.5M dioxane hydrochloride solution, react at 20°C for 4 hours, concentrate, add 100ml water and 100ml ethyl acetate, separate layers, add 20ml methanol in the water phase, and then Adjust the pH to 10-11 with 10% aqueous sodium hydroxide solution, grow crystals for 30 minutes, and filter to obtain 8.85 g of crude product, with a yield of 60%.
[0030] Suspend 8.0 g of the crude product in 80 ml of a mixed solvent (methanol: ethyl acetate = 1:3), heat to reflux until dissolved, naturally cool at 20°C and stir for 12 hours, and ...
Embodiment 2
[0032] Dissolve 10.0g (38.2mmol 1.0eq) of triphenylphosphine in 100ml of THF, cool at 0°C, add 7.67g (38.2mmol 1.0eq) of (s)-1-tert-butoxycarbonyl-3-hydroxypiperidine and 11.5g (38.2mmol 1.0eq) 3-(4-phenoxyphenyl)-1H-pyrazol[3,4-d]pyrimidin-4-amine, after stirring for 20 minutes, 7.67g (38.2mmol 1.0eq ) DIAD was added dropwise to the reaction liquid system, and kept at 0° C. for 20 hours. Then concentrate the feed solution to a viscous shape, add 380ml 0.5M dioxane hydrochloride solution, react at 20°C for 4 hours, concentrate, add 100ml water and 100ml ethyl acetate, separate layers, add 10ml ethanol in the water phase, and then Adjust the pH to 10-11 with 20% aqueous sodium hydroxide solution, grow crystals for 30 minutes, and filter to obtain 9.13 g of crude product, with a yield of 61.9%.
[0033] Suspend 8.0 g of the crude product in 64 ml of a mixed solvent (methanol: acetonitrile = 1:2), heat to reflux until dissolved, naturally cool at 20°C and stir for 12 hours, and ...
Embodiment 3
[0035] Dissolve 10.0g (38.2mmol 1.0eq) of triphenylphosphine in 100ml of THF, cool at 0°C, add 7.67g (38.2mmol 1.0eq) of (s)-1-tert-butoxycarbonyl-3-hydroxypiperidine and 11.5g (38.2mmol 1.0eq) 3-(4-phenoxyphenyl)-1H-pyrazol[3,4-d]pyrimidin-4-amine, after stirring for 20 minutes, 7.67g (38.2mmol 1.0eq ) DIAD was added dropwise to the reaction liquid system, and kept at 0° C. for 20 hours. Then concentrate the feed solution to a viscous shape, add 380ml 0.5M dioxane hydrochloride solution, react at 20°C for 4 hours, concentrate, add 100ml water and 100ml ethyl acetate, separate layers, add 15ml acetone in the water phase, and then Adjust the pH to 10-11 with 15% potassium hydroxide aqueous solution, grow crystals for 30 minutes, and filter to obtain 8.66 g of crude product, with a yield of 58.7%.
[0036] Suspend 8.0 g of the crude product in 56 ml of mixed solvent (methanol: ethanol = 1:1), heat to reflux until dissolved, naturally cool at 20°C and stir for 12 hours, and filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com