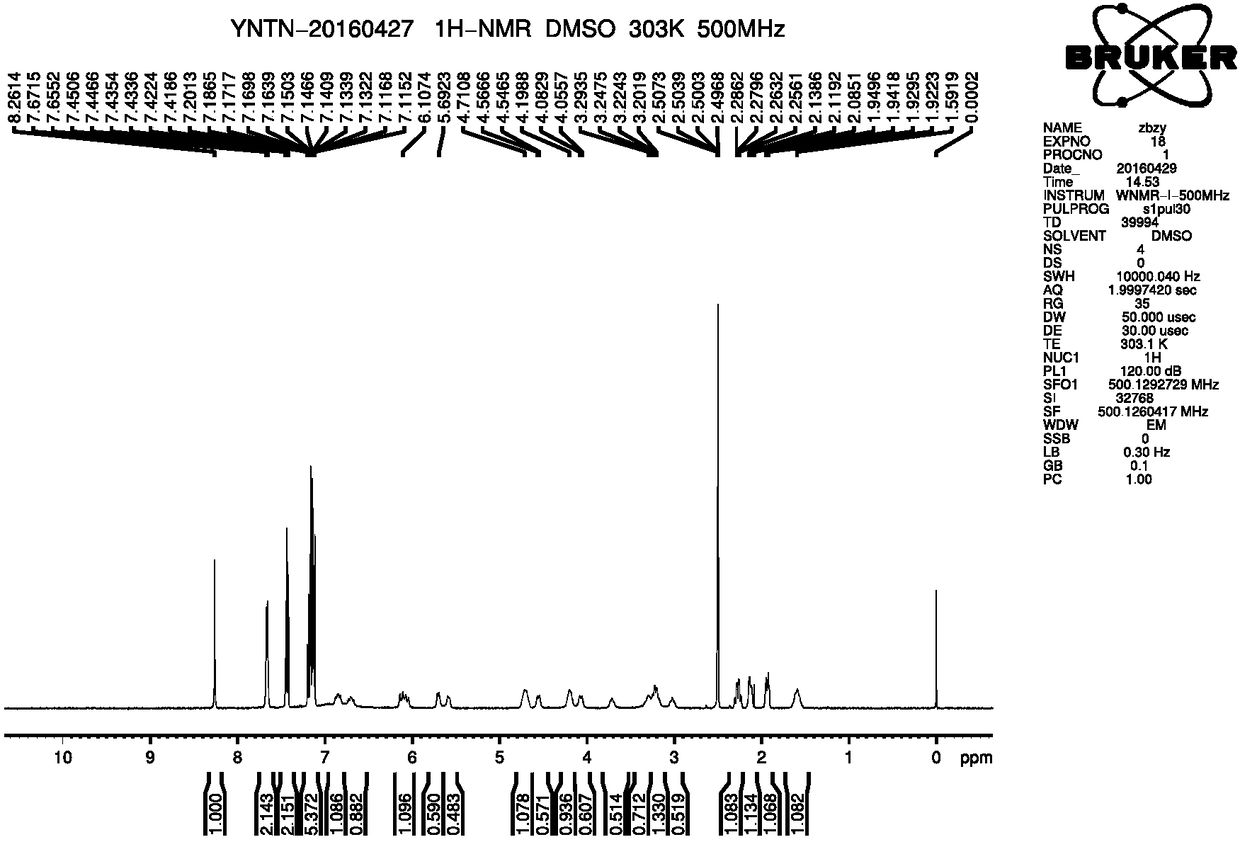
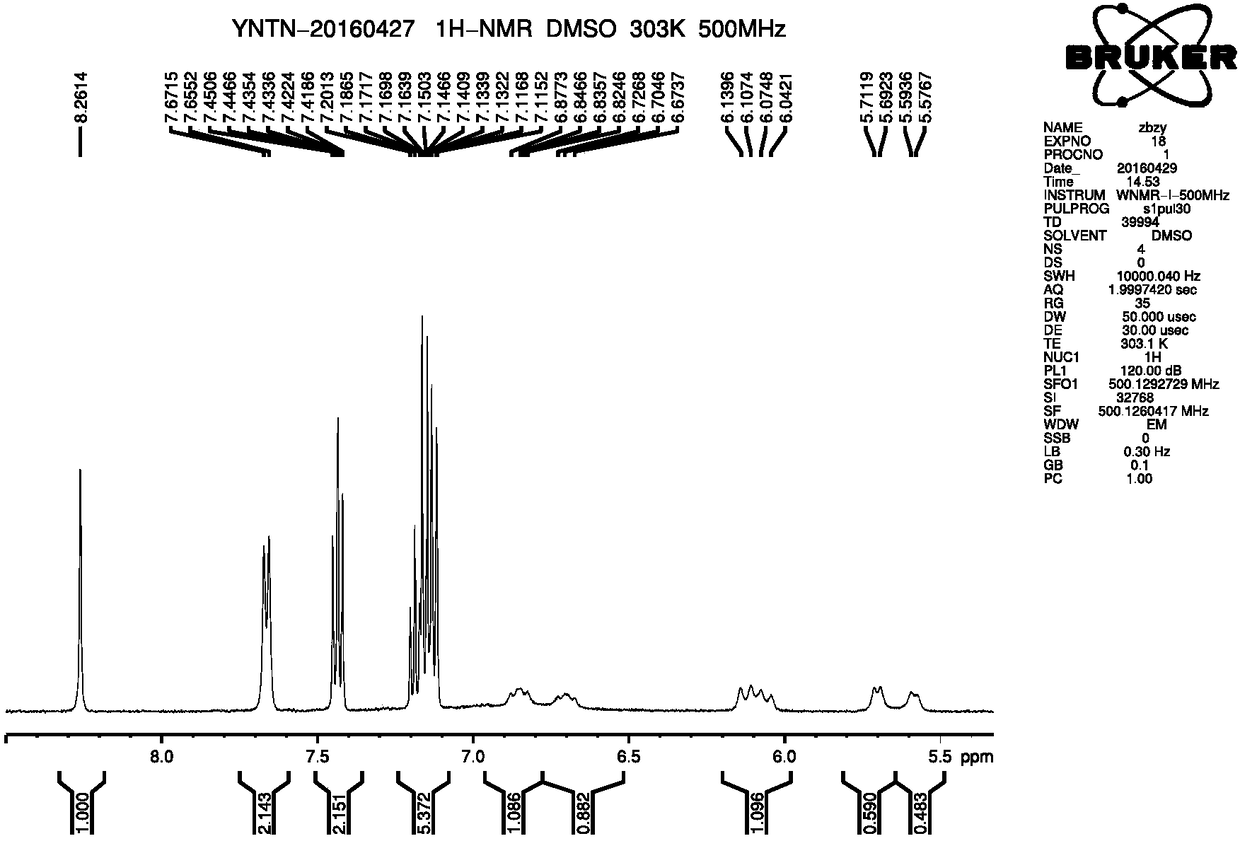
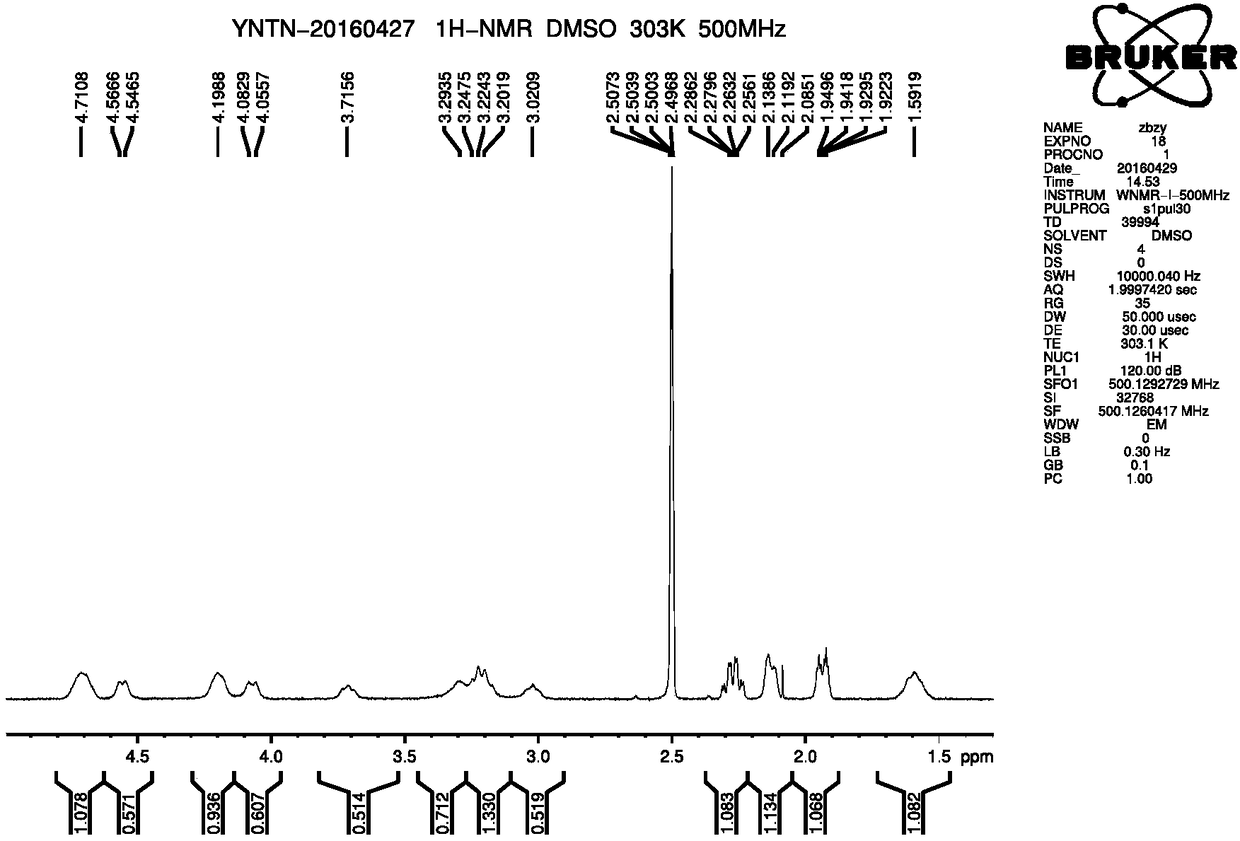
A kind of preparation method of ibrutinib
A technology of ibrutinib and intermediates, applied in the field of drug synthesis, can solve the problems of difficulty in industrialized production, many amidation by-products, low yield, etc. good rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]Preparation of Intermediate 1
[0046] 54.05g (0.5mol) of 3-amino-4-cyanopyrazole was added to a 1L reaction flask, 540mL of ethylene glycol monomethyl ether was added, and 52.06g (0.5mol) of formamidine acetate was added under the protection of nitrogen at room temperature, and the system was protected by nitrogen. React at 90°C for 48h. After the reaction was complete, the temperature was lowered to room temperature. At this time, a large amount of solids were precipitated in the system, filtered, and the filter cake was rinsed twice with 100 mL of methanol to obtain 61.9 g of the crude product intermediate. The crude product was recrystallized with 250 mL of toluene and 250 mL of acetic acid, filtered to obtain a ≥99% white solid 55.40 g, yield: 82.0%, HPLC: 99.9%.
[0047] Preparation of intermediate 2
[0048] Add 40.54g (0.3mol) of intermediate 1 and 360mL of DMF to a 1L reaction flask, and at the same time add 101.24g (0.45mol) of N-iodosuccinimide, and the syst...
Embodiment 2
[0059] Preparation of Intermediate 1
[0060] Add 54.05g (0.5mol) of 3-amino-4-cyanopyrazole to 1L reaction flask, 540mL of ethylene glycol monomethyl ether, add 62.47g (0.6mol) of formamidine acetate under nitrogen protection at room temperature, and nitrogen protection of the system, Reaction at 100°C for 46h. After the reaction was complete, the temperature was lowered to room temperature. At this time, a large amount of solids were precipitated in the system, filtered, and the filter cake was rinsed twice with 100mL methanol to obtain 63.8g of the crude intermediate. ≥99% white solid 56.41 g, yield: 83.5%, HPLC: 99.93%.
[0061] Preparation of Intermediate 2
[0062] Add 40.54g (0.3mol) of intermediate 1 and 360mL of DMF to a 1L reaction flask, and add 80.1g (0.45mol) of N-bromosuccinimide at the same time. The system is heated to 80°C for 22-24h, controlled by TLC, The raw material reacted completely. 360 mL of water was added to the system to quench the reaction, and...
Embodiment 3
[0070] Preparation of Intermediate 1
[0071] Add 54.05g (0.5mol) 3-amino-4-cyanopyrazole to 1L reaction flask, 540mL ethylene glycol monomethyl ether, add 67.68g (0.65mol) formamidine acetate at room temperature under nitrogen protection, and nitrogen protection system, Reaction at 120°C for 45h. After the reaction was complete, the temperature was lowered to room temperature. At this time, a large amount of solids were precipitated in the system, filtered, and the filter cake was rinsed twice with 100mL methanol to obtain 62.2g of the crude intermediate. ≥99% white solid 56.82g, yield: 84.1%, HPLC: 99.91%.
[0072] Preparation of Intermediate 2
[0073] Add 40.54g (0.3mol) of intermediate 1 and 360mL of DMF to a 1L reaction flask, and at the same time add 101.24g (0.45mol) of N-iodosuccinimide, and the system is heated to 80°C for 22-24h, controlled by TLC. The raw material reacted completely. 360 mL of water was added to the system to quench the reaction, and a large am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com