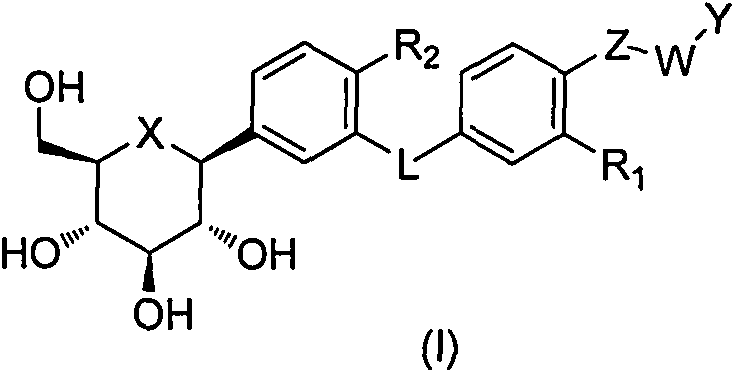
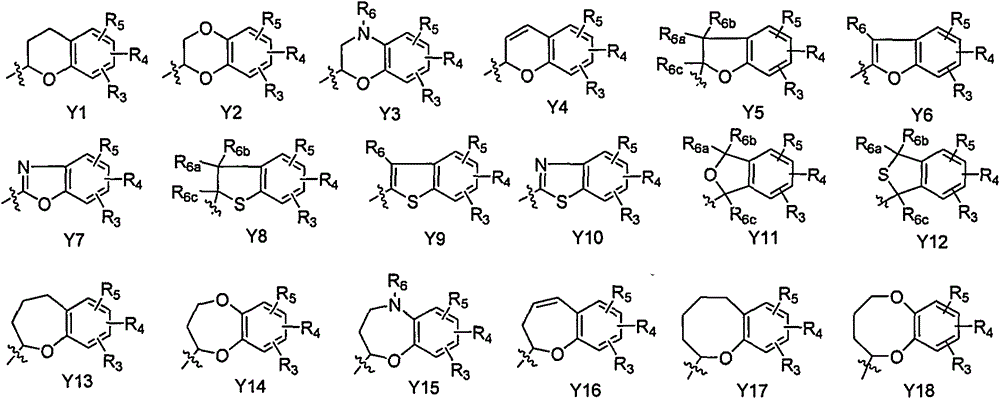
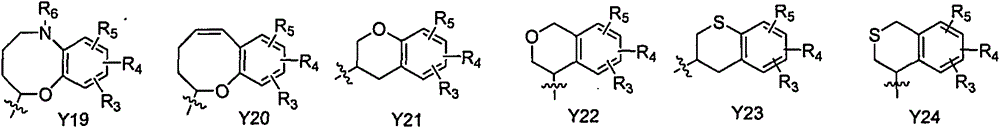
C-aryl glucoside derivative, as well as medical composition, preparation method and application thereof
A technology of aryl glycosides and derivatives, applied in the field of C-aryl glycoside derivatives, can solve problems such as adverse reactions, hypoglycemia, decreased insulin secretion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] Example 1: Synthesis of 2,3,4,6-tetra-O-(trimethylsilyl)-D-glucopyranone
[0142]
[0143] D-glucono δ-lactone (100.0g, 0.56mol) was dissolved in tetrahydrofuran (700mL), triethylamine (511g, 5.05mol) was added dropwise under ice-cooling and trimethylchlorosilane (427g, 3.93mol ) for about 1 hour, after the dropwise addition was completed, stir in an ice bath for 2 hours, and the system rose to room temperature and stirred overnight. Add ethyl acetate (2L), wash with saturated aqueous sodium dihydrogen phosphate (1L×2), water (1L) and saturated brine (1L×2) respectively, dry the organic phase with anhydrous magnesium sulfate, filter, and The solvent was rotary evaporated under reduced pressure to obtain a colorless oil, added toluene (100mL×2), and distilled under reduced pressure to constant weight to obtain 2,3,4,6-tetra-O-(trimethylsilyl)-D-pyran Glucosone (254 g, 97% yield) was a colorless liquid.
[0144] 1 HNMR (400MHz, CDCl 3 ): δ4.17-4.20(m, 1H), 4.01(d, ...
Embodiment 2
[0146] Embodiment 2: the synthesis of 5-bromo-2-cyanobenzoic acid
[0147]
[0148] Dissolve diisopropylamine (6.67g, 65.9mmol) in tetrahydrofuran (30mL) under nitrogen protection, cool to -30°C, and slowly add n-butyllithium in n-hexane (2.5M, 26.3mL, 65.9mmol ). The reaction system was stirred at -30°C for 30 minutes, then cooled to -70°C, p-bromophenylacetonitrile (10 g, 54.9 mmol) was slowly added dropwise, kept at -70°C for 30 minutes, and then solid carbon dioxide was added. The reaction system was slowly raised to room temperature and stirred overnight. Add water (50mL) and ethyl acetate (100mL), separate the organic phase, extract the aqueous phase with ethyl acetate (100mL×2), combine the organic phases, wash with saturated brine, dry over anhydrous magnesium sulfate, filter, reduce The solvent was removed by rotary evaporation to give 5-bromo-2-cyanobenzoic acid (7.25 g, yield: 58%) as a white solid.
[0149] m / z: [M-H] - 225.9
Embodiment 3
[0150] Embodiment 3: the synthesis of 5-bromo-2-methoxybenzoic acid
[0151]
[0152] 2-Methoxy-5-bromobenzaldehyde (20g, 93.0mmol) was dissolved in acetone (100mL), and after cooling to 5°C, solid potassium permanganate (14.7g, 93.0mmol) was added in portions within 2 hours . After the addition was complete, the reaction system was stirred overnight at room temperature. The mixture was filtered, and the filtrate was adjusted to pH=1 with 5M hydrochloric acid, and a pale yellow solid was precipitated, which was filtered. After vacuum drying, 5-bromo-2-methoxybenzoic acid (10.5 g, yield: 49%) was obtained.
[0153] m / z: [M-H] - 228.9
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com