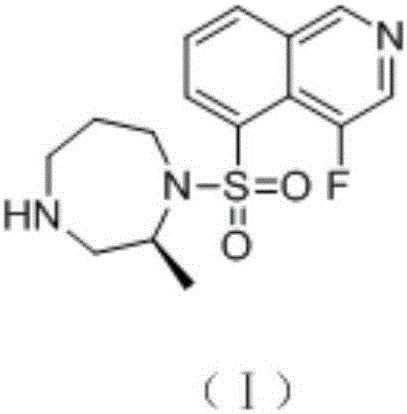
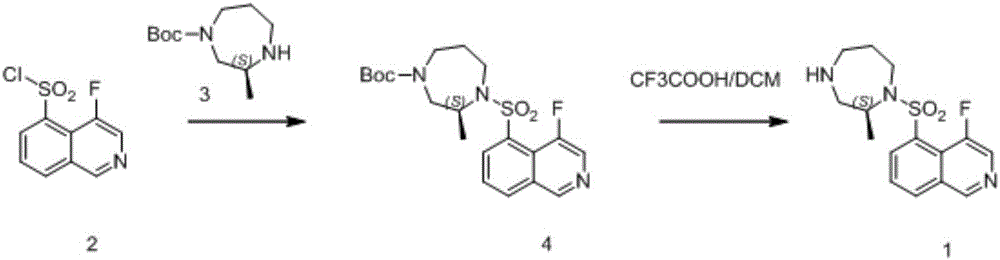
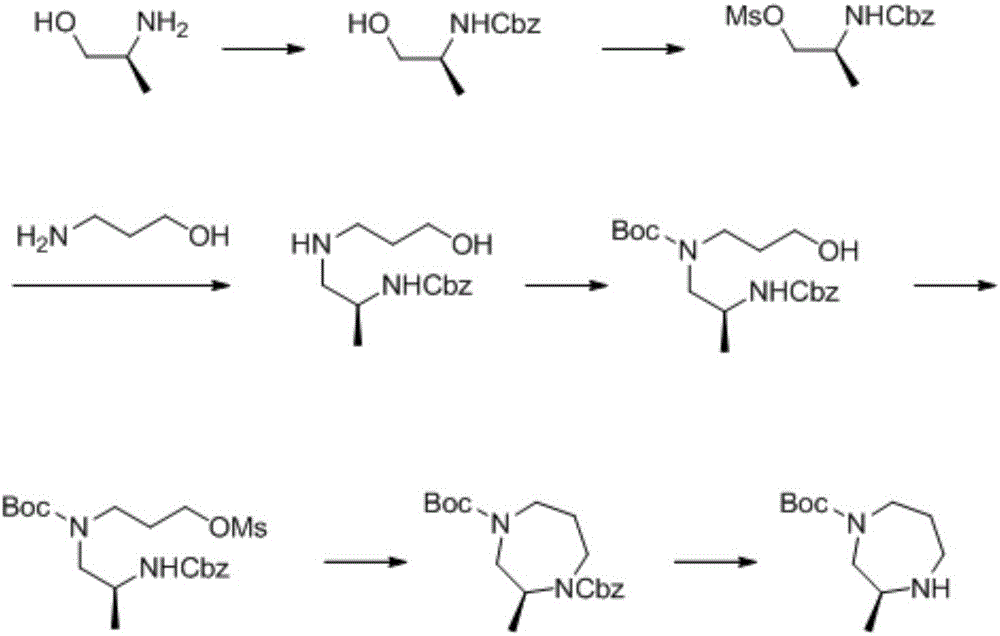
Preparation method of 1,4-dioazo-cycloheptane derivative
A technology for diazepane and derivatives is applied in the field of drug synthesis, which can solve the problems of poor stability, low total yield, and affect reproducibility, and achieves the effects of mild reaction conditions, short synthetic route, and optimized operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A preparation method of (S)-(-)-1-(4-fluoroisoquinolin-5-yl)sulfonyl-2-methyl-1,4-diazepane, the steps of which are:
[0039] 1) 2-(S)-1-(4-fluoroisoquinoline-5-sulfinylamino)-N-(tert-butoxycarbonyl)-N-(3-tert-butyldimethylsilyloxy The preparation of propyl) propylamine:
[0040] Dissolve 2.3g (6.72mmol) of 2-(S)-2-amino-N-(tert-butoxycarbonyl)-N-(3-tert-butyldimethylsilyloxypropyl)propylamine in 15ml of dichloromethane After cooling down to -5-0°C, add 0.74g (7.33mmol) triethylamine and 0.15g 4-dimethylaminopyridine, slowly add 1.5g (6.1mmol) 4-fluoroisoquinoline-5 under stirring - Sulfonyl chloride, react at -5-0°C for 45min, then rise to 20-30°C, react at 20-30°C for 16h, after the reaction is completed, add 10mL of water to the resulting system after the reaction, and extract with 8ml of dichloromethane 3 times, after the extraction was completed, the resulting organic phase was dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated an...
Embodiment 2
[0074] A preparation method of (S)-(-)-1-(4-bromoisoquinolin-5-yl)sulfonyl-2-methyl-1,4-diazepane, the steps of which are:
[0075] 1) 2-(S)-1-(4-bromoisoquinoline-5-sulfinylamino)-N-(tert-butoxycarbonyl)-N-(3-tert-butyldimethylsilyloxy The preparation of propyl) propylamine:
[0076] Dissolve 1.87g (5.38mmol) of 2-(S)-2-amino-N-(tert-butoxycarbonyl)-N-(3-tert-butyldimethylsilyloxypropyl)propylamine in 5ml of dichloromethane After cooling down to -5-0°C, 1.73g (17.13mmol) of triethylamine was added thereto, and 1.5g (4.89mmol) of 4-bromoisoquinoline-5-sulfonyl chloride was slowly added under stirring. After reacting at ℃ for 45min, rise to 20-30℃, and react at 20-30℃ for 16h. After the reaction is completed, add 8mL of water to the system obtained after the reaction, and extract 3 times with 6-8ml of dichloromethane. After the extraction is completed, , the resulting organic phase was dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated and dri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com