Preparation method of drug intermediate (S)-3-hydroxytetrahydrofuran
A hydroxytetrahydrofuran and tetrahydrofuran-based technology is applied in the field of synthesis of a pharmaceutical intermediate-3-hydroxytetrahydrofuran, and can solve problems such as low overall yield, unsuitability for large-scale production, and failure to solve the problem of using intermediates with opposite configurations. , to achieve the effect of high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
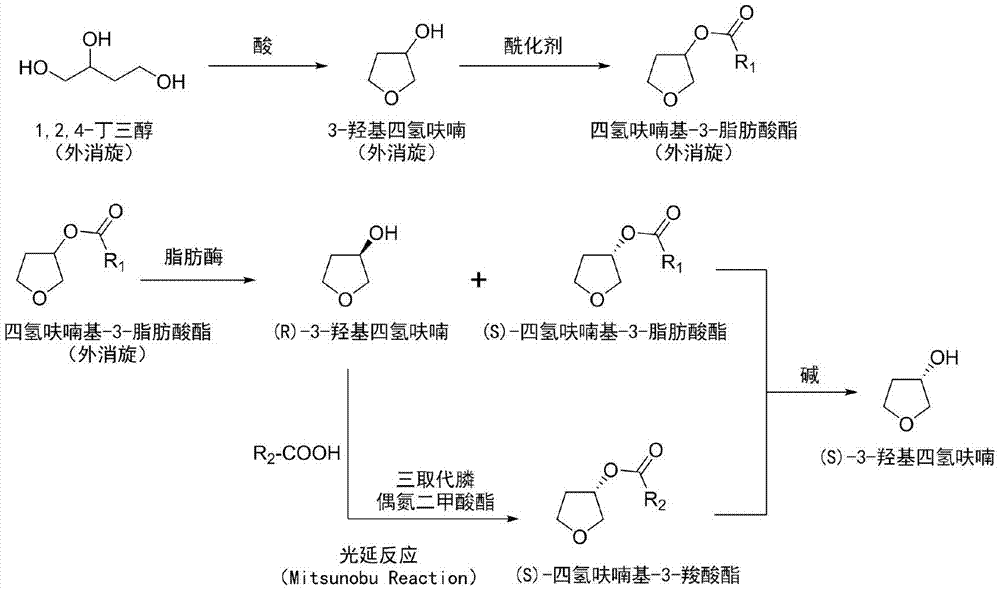
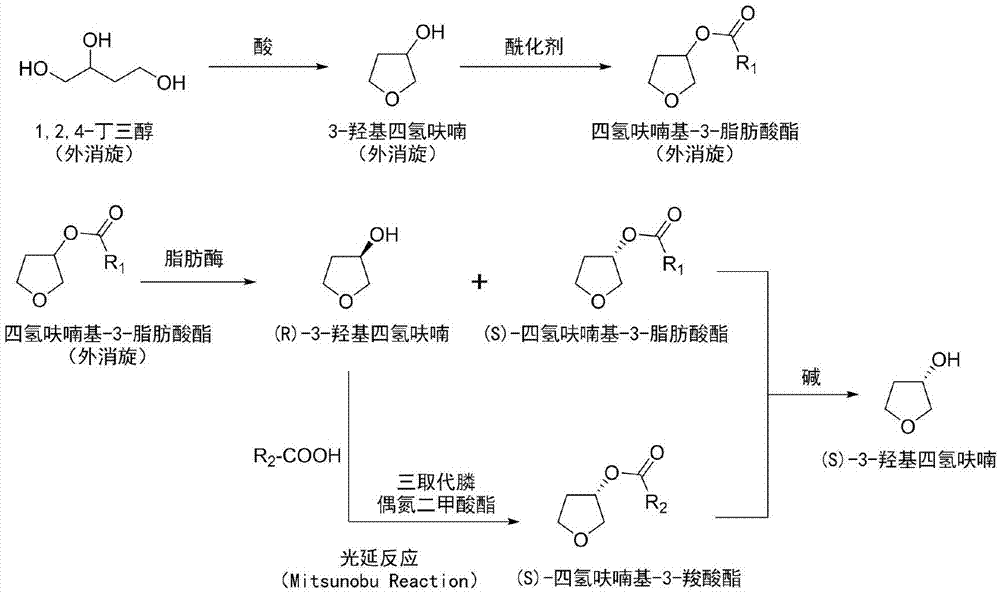
[0041] Add 1.06kg (10mol) of 1,2,4-butanetriol to a 5L reactor, add a catalytic amount of p-toluenesulfonic acid monohydrate 19g (0.1mol) under stirring, and heat to dissolve it completely in 1,2 , 4-Butanetriol. Under reduced pressure (1.6kPa), the temperature was raised to 90°C, and the 80-90°C fraction was collected by a distillation device to obtain 3-hydroxytetrahydrofuran as a colorless liquid. The collected 3-hydroxytetrahydrofuran was dissolved in an appropriate amount of anhydrous dichloromethane, added molecular sieves to dry and filtered, and the dichloromethane was distilled off under reduced pressure to obtain 678 g of anhydrous 3-hydroxytetrahydrofuran with a yield of 77%.
[0042] In a 25L reactor, 678g (7.7mol) of 3-hydroxytetrahydrofuran was dissolved in 8L of anhydrous dichloromethane, and then 1.13L (14mol) of anhydrous pyridine was added. The reaction solution was cooled to 0°C, and a solution of acetyl chloride (600ml, 8.4mol) in dichloromethane (2L) was ...
Embodiment 2
[0046] 10kg of racemic 1,2,4-butanetriol was reacted with 190g of p-toluenesulfonic acid monohydrate, and after vacuum distillation, 6.6kg of racemic 3-hydroxytetrahydrofuran was obtained. Dissolve all the obtained 3-hydroxytetrahydrofuran (racem) in 50L of dichloromethane, add 11L of anhydrous pyridine, and slowly add 6L of acetyl chloride after cooling to 0°C. After the reaction, 8.6kg of tetrahydrofuranyl-3-ethane Ester (racemic). Catalyzed hydrolysis of all tetrahydrofuryl-3-hexanoate (racemic) by 10 g lipase A lyophilized powder aqueous solution, after the reaction, (S)-tetrahydrofuryl-3-hexanoate and (R)-3- Mixtures of hydroxytetrahydrofuran. Dissolve the treated mixture in anhydrous tetrahydrofuran, add 11.7kg triphenylphosphine and 7.5kg p-nitrobenzoic acid, cool to 0-4°C, add 9.5L diisopropyl azodicarboxylate while stirring, and continue Stir until the reaction is complete. Add 10 L of sodium hydroxide solution (1N) to the reaction solution, stir at room temperatur...
Embodiment 3
[0048] Take 5 kg of racemic 1,2,4-butanetriol, add 0.5 L of concentrated hydrochloric acid to react, and obtain 3.3 kg of racemic 3-hydroxytetrahydrofuran after vacuum distillation. All the obtained 3-hydroxytetrahydrofuran (racemic) was dissolved in 20L of dichloromethane, 5.6L of anhydrous pyridine was added, and after cooling to 0°C, 3.8L of acetic anhydride was slowly added. After the reaction, 4.15 kg of tetrahydrofuranyl-3-acetate (racem) was obtained. Catalyzed hydrolysis of all tetrahydrofuranyl-3-acetate (racemic) by 4g lipase A lyophilized powder aqueous solution, after the reaction, (S)-tetrahydrofuryl-3-acetate and (R)-3- Mixtures of hydroxytetrahydrofuran. Dissolve the treated mixture in anhydrous tetrahydrofuran, add 6.5kg triphenylphosphine and 1.5L glacial acetic acid, cool to 0-4°C, add 5L diethyl azodicarboxylate with stirring, and continue stirring until the reaction is complete. Add 5L of sodium hydroxide solution (1N) to the reaction solution, stir at ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com