Novel technology for trans-2,6-lupetazin
A technology of dimethylpiperazine and a new process, applied in the field of pharmaceutical intermediates, can solve the problems of high temperature, many inorganic salts, and high reaction temperature, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
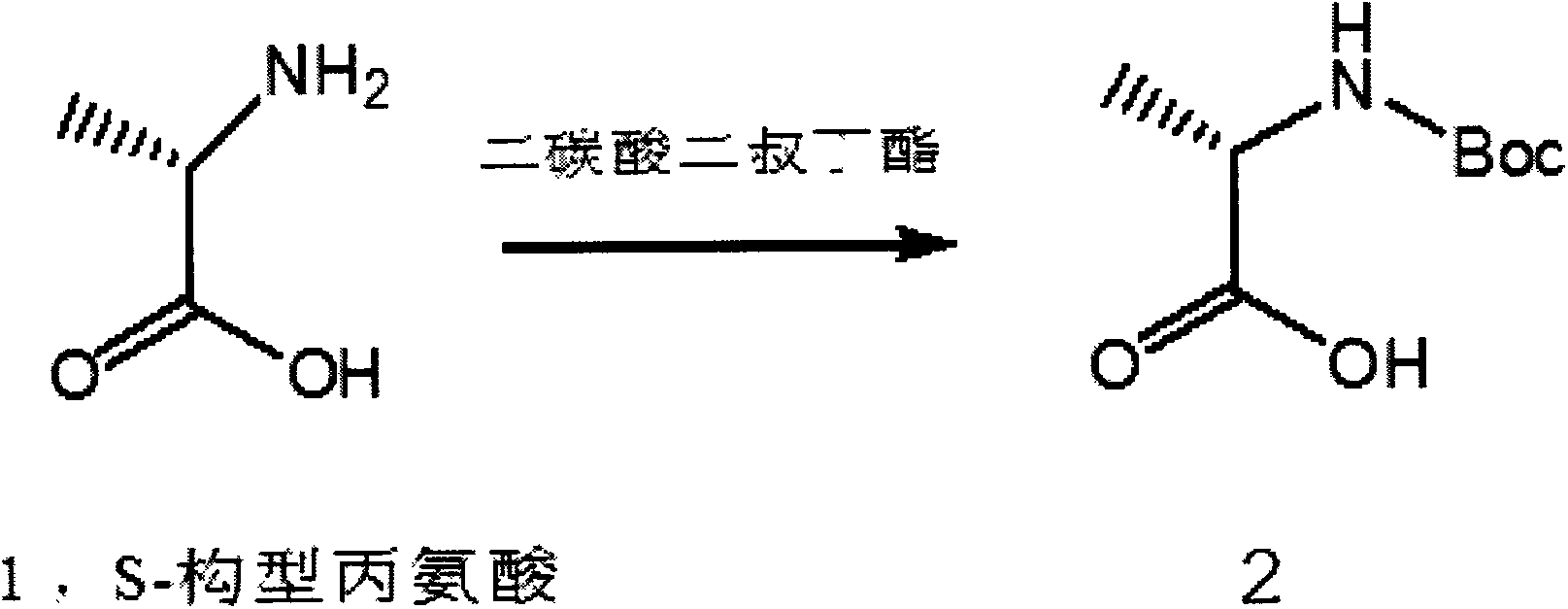
[0028] Using the S-configuration alanine as the starting material, adding di-tert-butyl dicarbonate, and using the two to make the di-tert-butyl dicarbonate carry out amino protection reaction on the S-configuration alanine, thereby Intermediate 2 is generated, and after purification treatment, the purification treatment here is chromatographic column purification, and the purity of intermediate 2 is further obtained to be more than 98%. In this process, the reaction temperature is 25 degrees Celsius, and the reaction time is 24 hours, or the temperature It can be carried out at room temperature instead of high temperature. The chemical reaction formula of this step is:
[0029]
[0030] Here 2 is intermediate 2, S-configuration alanine is left-handed configuration alanine, S means left, that is, counterclockwise rotation.
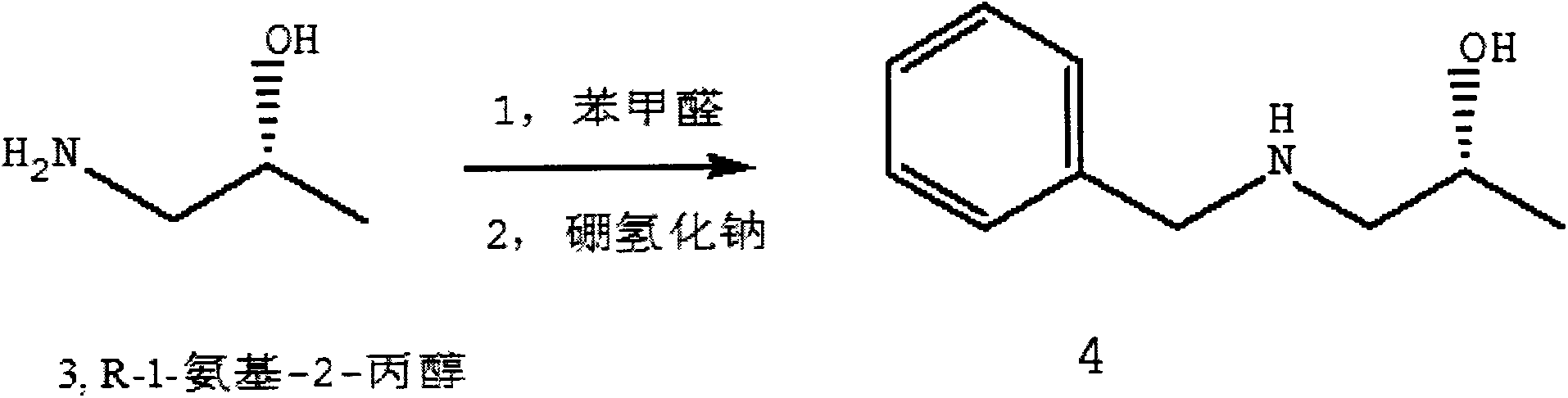
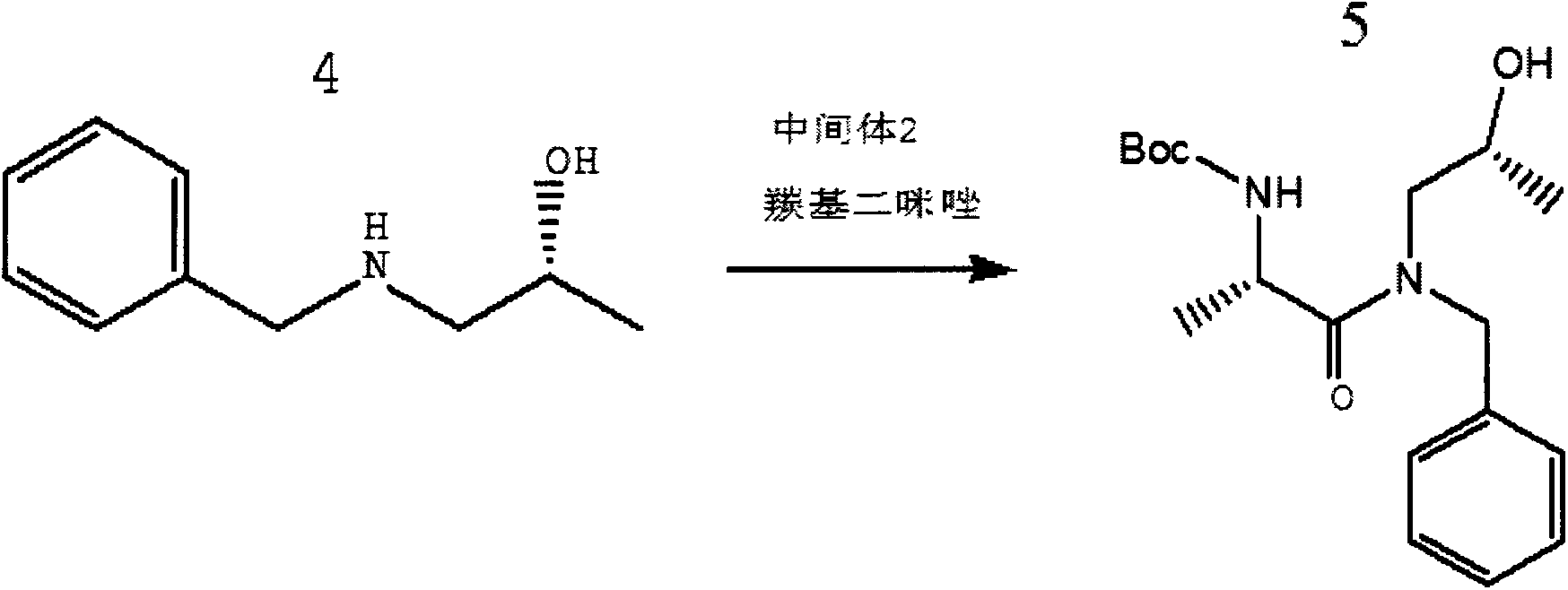
[0031] Using R-1-amino-2-propanol as a raw material, adding benzaldehyde, and using the two to condense the amino group on the R-1-amino-2-propanol wit...
Embodiment 2
[0043] With R-configuration alanine as starting material, add di-tert-butyl dicarbonate, and make the di-tert-butyl dicarbonate carry out amino protection reaction on the R-configuration alanine, thereby Intermediate 2 is generated, and after purification treatment, the purification treatment here is chromatographic column purification, and the purity of intermediate 2 is further obtained to be more than 98%. In this process, the reaction temperature is 25 degrees Celsius, and the reaction time is 24 hours, or the temperature It can be carried out at room temperature instead of high temperature. The chemical reaction formula of this step is:
[0044]
[0045] Here 2 is intermediate 2, R-configuration alanine is right-handed configuration alanine, R means right, ie clockwise rotation.
[0046] Using S-1-amino-2-propanol as a raw material, add benzaldehyde, and make the amino group on the S-1-amino-2-propanol condense with the carbonyl on the benzaldehyde, And sodium borohyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com