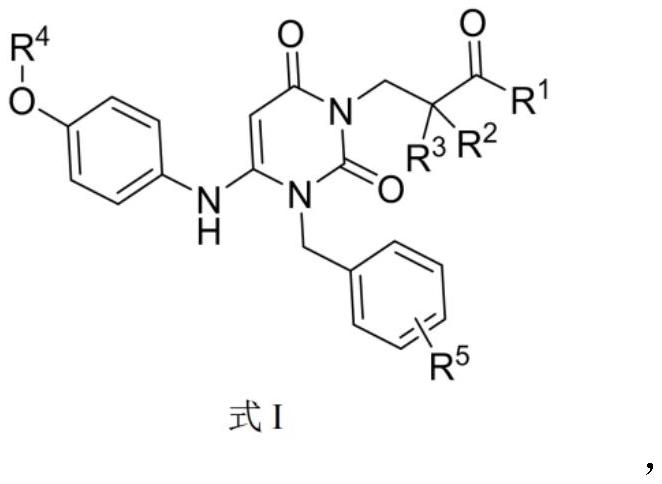
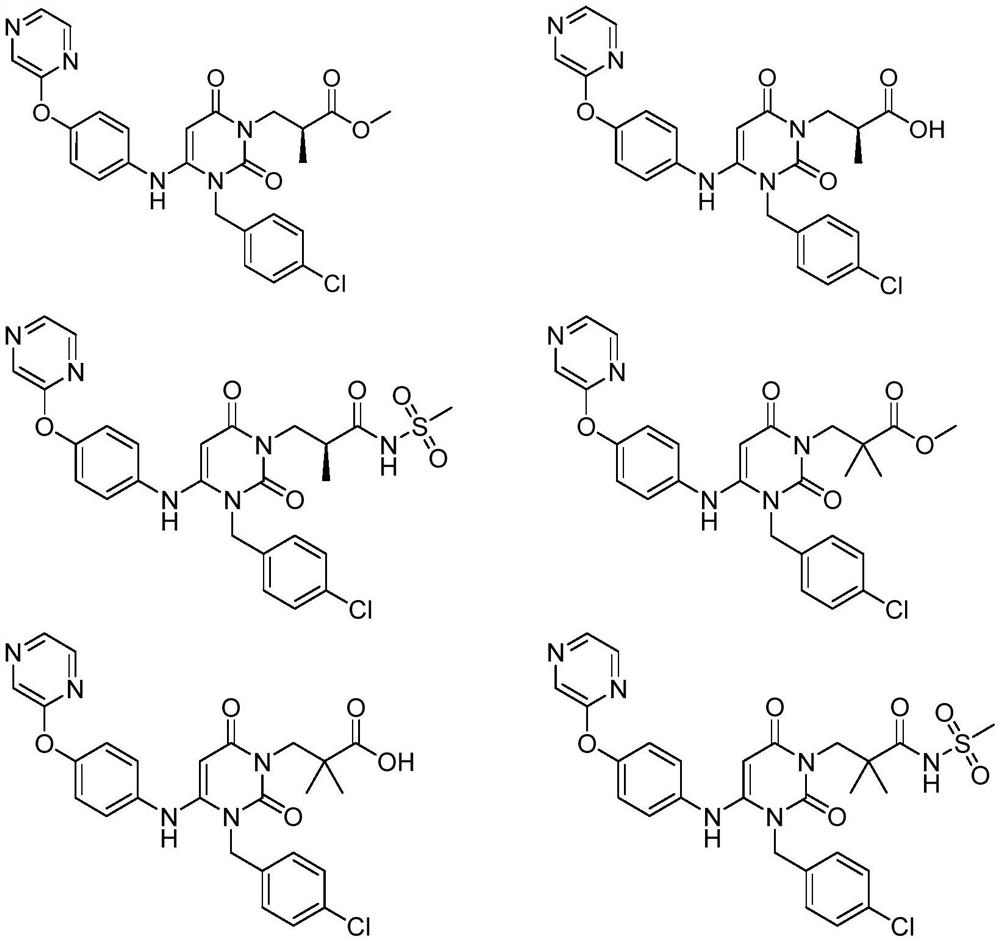
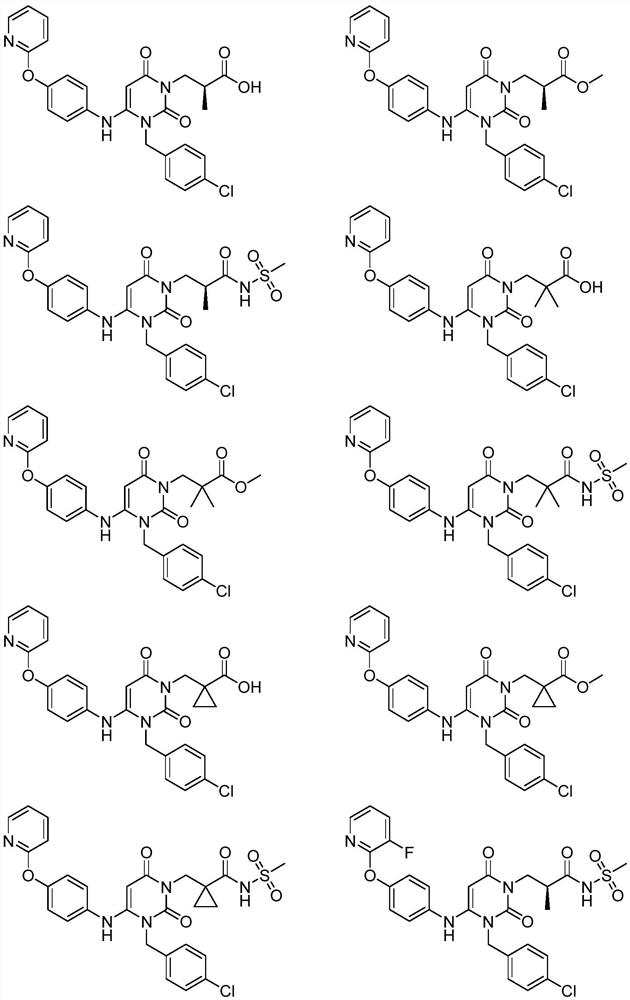
Dihydropyrimidine compound as well as preparation method and application thereof
A compound and solvate technology, applied in the field of medicinal chemistry, can solve the problems of high incidence of taste-related adverse events and failure to reach the main efficacy endpoint, and achieve good P2X3 receptor antagonism, good inhibitory effect, and long-term effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: (S)-3-(3-(4-chlorobenzyl)-2,6-dioxo-4-(4-(pyrazine-2-oxyl)phenyl)amino)-3, Preparation of 6-dihydropyrimidin-1(2H)-yl)-2-methylpropanoic acid methyl ester (compound 1):
[0067]
[0068] Step 1: Preparation of 4-(pyrazine-2-oxyl)aniline (compound b-1)
[0069] 2-Fluoropyrazine (50.0g, 0.52mol) and p-aminophenol (53.5g, 0.49mol) were dissolved in dimethylsulfoxide (360ml), cesium carbonate (320g, 0.98mol) was added to obtain a reaction mixture, and mechanically Stir the reaction mixture at constant speed. Subsequently, the internal temperature of the reaction system was raised to 80° C. for 2-3 hours. The progress of the reaction was tracked by thin-layer chromatography. After the reaction was complete, the reaction mixture was added to three times the volume (about 1 L) of water and kept stirring. Then extract the product three times with ethyl acetate, combine and dry the ethyl acetate and concentrate to obtain the crude product. The crude product is s...
Embodiment 2
[0081] Example 2: S-3-(3-(4-chlorobenzyl)-2,6-dioxo-4-(4-(pyrazine-2-oxyl)phenyl)amino)-3,6- Preparation of dihydropyrimidin-1(2H)-yl)-2-methylpropionic acid (compound 2)
[0082] (S)-3-(3-(4-chlorobenzyl)-2,6-dioxo-4-(4-(pyrazine-2-oxyl)phenyl) prepared by the method in Example 1 Amino)-3,6-dihydropyrimidin-1(2H)-yl)-2-methylpropionic acid methyl ester (522mg, 1.0mmol) was dissolved in a mixed solvent of methanol (3ml) and tetrahydrofuran (3ml), kept At a temperature around 10°C, a solution of lithium hydroxide (168mg, 4mmol) in water (3ml) was added to obtain a reaction mixture which was allowed to react overnight at RT. Thin-layer chromatography followed the reaction process. After the reaction was complete, purified by column chromatography to obtain S-3-(3-(4-chlorobenzyl)-2,6-dioxo-4-(4-(pyrazine-2- Oxy)phenyl)amino)-3,6-dihydropyrimidin-1(2H)-yl)-2-methylpropionic acid (388 mg, off-white solid), yield: 76.5%, purity: 98.62%.
[0083] ESI-MS:m / z=508.1(M+H) + .
[00...
Embodiment 3
[0085] Example 3: (S)-3-(3-(4-chlorobenzyl)-2,6-dioxo-4-(4-(pyrazine-2-oxyl)phenyl)amino)-3, Preparation of 6-dihydropyrimidin-1(2H)-yl)-2-methyl-N-(methylsulfonyl)propionamide (compound 3)
[0086] The S-3-(3-(4-chlorobenzyl)-2,6-dioxo-4-(4-(pyrazine-2-oxyl)phenyl)amino obtained by the method of Example 2 )-3,6-dihydropyrimidin-1(2H)-yl)-2-methylpropionic acid (compound 2) (288mg, 0.57mmol), DIPEA (183mg, 1.42mmol) and EDCI (130.8mg, 0.68mmol ), dissolved with dichloromethane (5ml), stirred for 15min, added methanesulfonamide (64.7mg, 0.68mmol) and DMAP (83mg, 0.68mmol) and reacted at room temperature for 2-3 hours, followed by TLC After the reaction is complete, the reaction mixture is washed with water, extracted three times with dichloromethane, combined, dried and concentrated, and purified by column chromatography to obtain (S)-3-(3-(4-chlorobenzyl)-2,6-dioxo-4 -(4-(pyrazine-2-oxyl)phenyl)amino)-3,6-dihydropyrimidin-1(2H)-yl)-2-methyl-N-(methylsulfonyl)propanamide ( 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com