Method for synthesizing and preparing 5'-S-(4, 4'-dimethoxytriphenylmethyl)-2'-deoxyinosine
A technology of dimethoxytrityl and dimethoxytrityl mercaptan, applied in the field of chemical synthesis, can solve the problems of being unsuitable for industrialized production, high production cost, low yield and the like, and achieves low price , low cost and high yield
Active Publication Date: 2013-05-01
NAT INST OF PHARMA R & D CO LTD
View PDF4 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
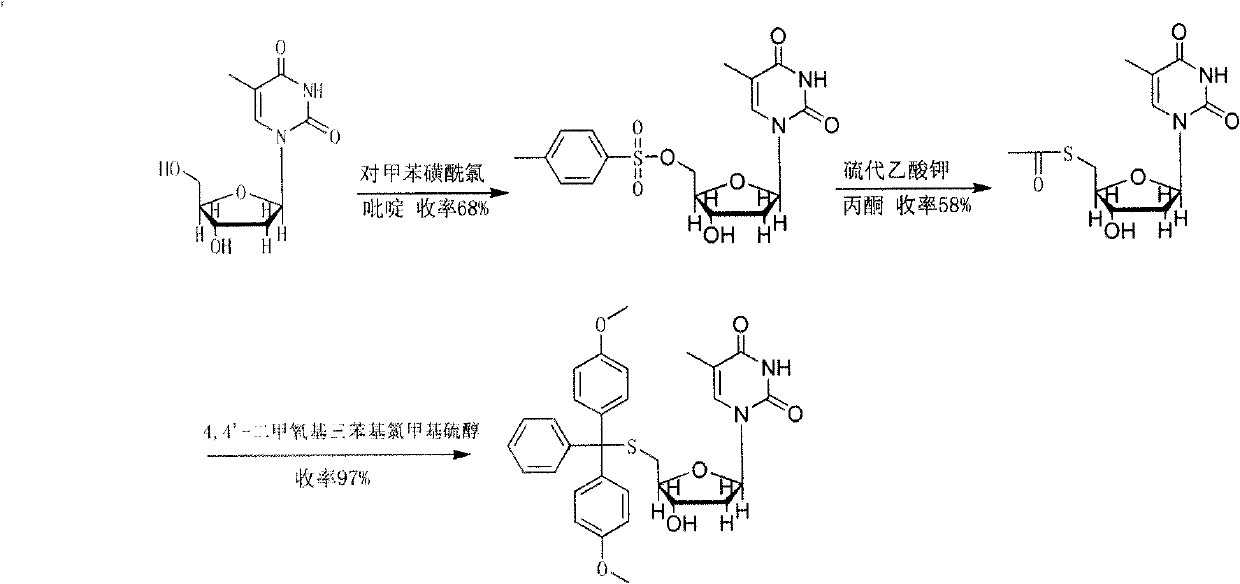
By activating the 5'-hydroxyl of 2'-deoxythymidine with methanesulfonyl chloride, after esterification with potassium thioacetate, 4,4'-dimethoxytriphenylmethylthiol ((MeO) 2 TrSH) (Efficient Solid Synthesis of Cleavable Oligodeoxynucleotides Based on a Novel Strategy for the Syhthesis of 5'-S-(4,4'-Dimethoxytrityl)-2'-deoxy-5'-thionucleoside Phosphramidites, Kerstin Jahn-Hofmann and Ioachin W.Engels, Helvetica Chimica Acta-Vol.87(2004), 2812-2828)), yet this method produces multiple intermediate products through multi-step reactions, and finally obtains 5'-S-(4,4'- The yield of dimethoxytrityl)-2'-deoxythymidine is on the low side, can only reach 38.3%, and wherein the third step has used 1,1,3,3-tetramethylguanidine as catalyst, Expensive, high production cost, not suitable for industrial production
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
preparation example Construction
Embodiment 1-5
Embodiment 6-10
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
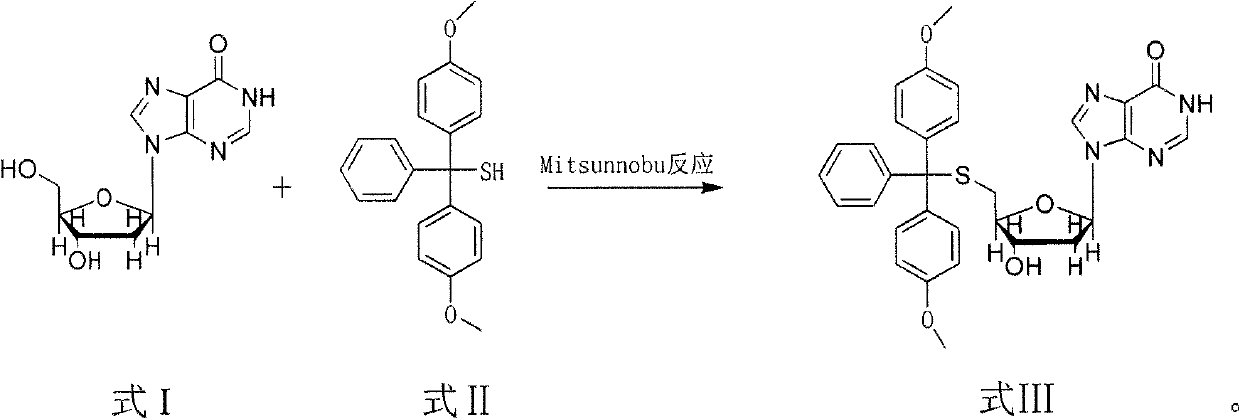
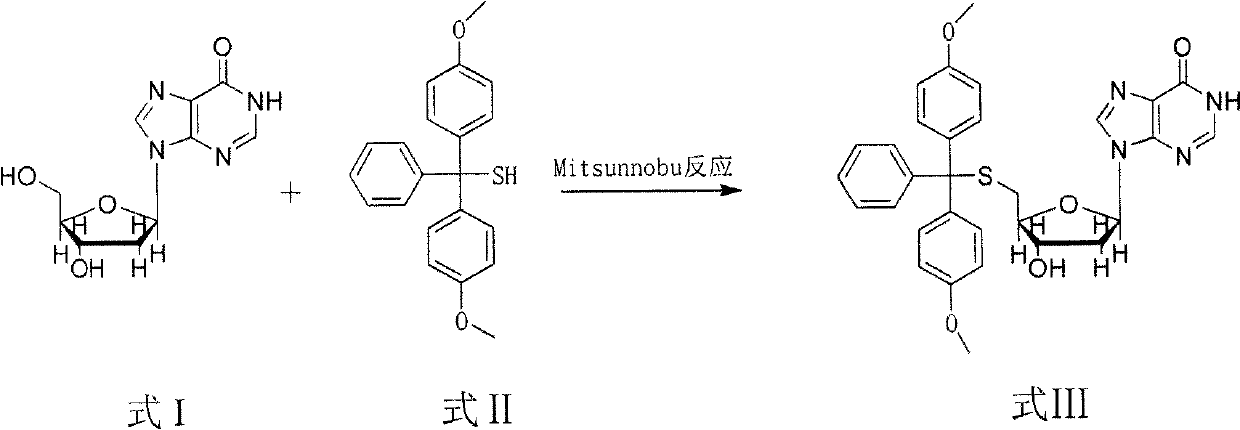
The invention discloses a method for synthesizing and preparing 5'-S-(4, 4'-dimethoxytriphenylmethyl)-2'-deoxyinosine. The method comprises the following steps of: under inert gas protection, carrying out mitsunobu reaction on 2'-deoxyinosine and 4, 4'-dimethoxytriphenylmethyl mercaptan in an organic solvent at room temperature under effects of an azo reagent, a phosphine coordination compound, organic alkali or protonic acid, i.e. carrying out intermolecular dehydration reaction on 5'-hydroxyl of the 2'-deoxyinosine and the 4, 4'-dimethoxytriphenylmethyl mercaptan to obtain a C-S bond, and obtaining the 5'-S-(4, 4'-dimethoxytriphenylmethyl)-2'-deoxyinosine. The method for synthesizing and preparing the 5'-S-(4, 4'-dimethoxytriphenylmethyl)-2'-deoxyinosine has the beneficial effects of being wide in raw material sources, low in price, high in yield, mild in reaction condition, good in selectivity, simple in operation process, convenient in post-treatment, and suitable for large-scale industrial production.
Description
technical field The invention relates to a synthesis and preparation method of 5'-S-(4,4'-dimethoxytrityl)-2'-deoxyinosine in the field of chemical synthesis. Background technique With the development of molecular biology and pharmaceutical industry, the research and development of nucleoside compounds and their derivatives has become a hot spot. With the deepening of research, the new nucleoside compounds obtained after functional group transformation and modification of nucleoside compounds and their derivatives are useful in the treatment of cardiovascular diseases, central nervous system diseases, circulatory and urinary system diseases, as well as antiviral and antitumor. It has a special curative effect and is widely used in molecular biology, medicine and other fields. Structural modification of nucleoside compounds and the introduction of heteroatom sulfur can sometimes greatly improve the antiviral activity of drugs, and at the same time reduce the toxic and side e...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07H19/173C07H1/00
Inventor 于中生魏可贵
Owner NAT INST OF PHARMA R & D CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com