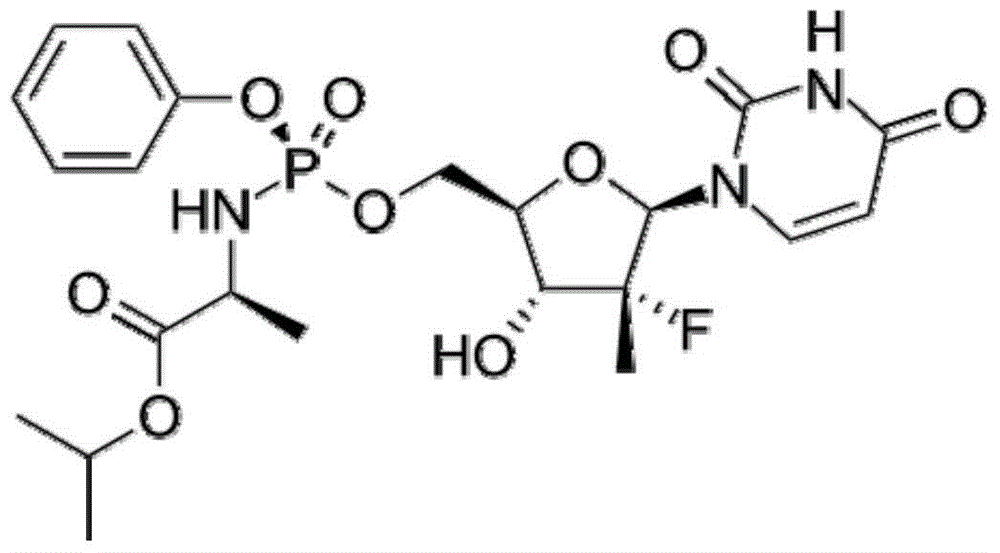
Method for preparing crystalline form 6 of Sofosbuvir
A technology of febuvir crystal form and crystal form, which is applied in the field of medicinal chemistry, can solve the problems that crystal form 6 cannot be stably obtained, the process conditions are difficult to control, and the crystallization process takes a long time to achieve easy implementation and good reproducibility , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
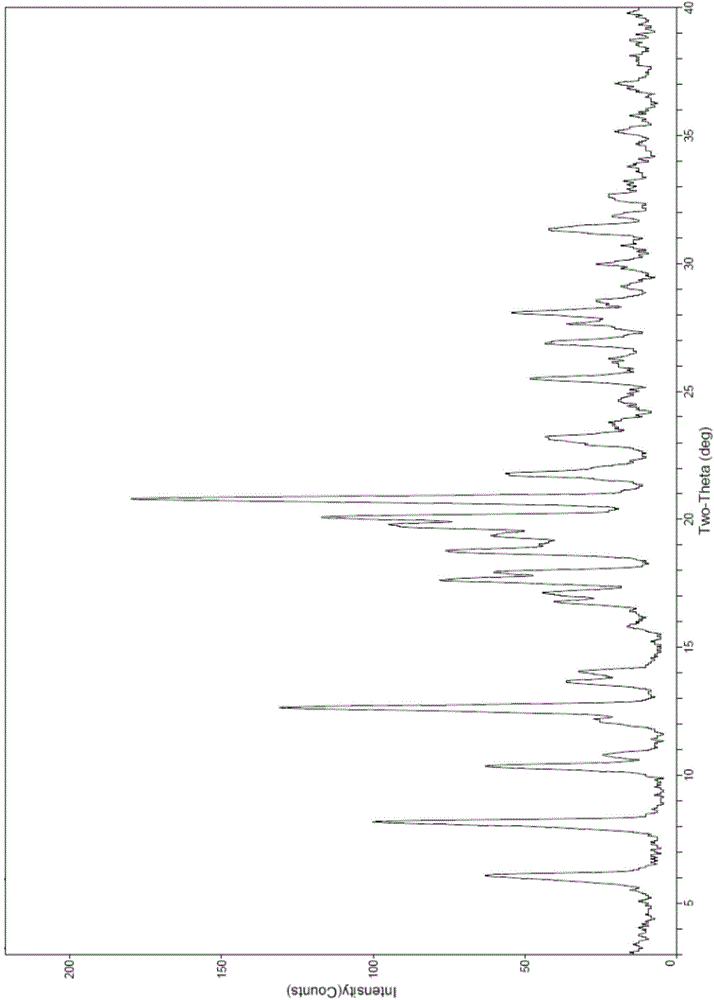
[0023] Dissolve 10 grams of sofosbuvir in 100 ml of methanol, add the solution to 300 ml of pure water preheated to 45°C, drop the temperature to -15°C and stir for 2 to 3 hours after the addition is complete, filter, and filter cake Vacuum drying at 60° C. to constant weight yielded 9.1 g of a white solid with a yield of 91% and a purity of 99.7%. The 2θ angles of the X-ray powder diffraction pattern of the prepared product are at 6.1°, 8.2°, 10.4°, 12.7°, 17.1°, 17.6°, 18.0°, 18.8°, 19.4°, 19.8°, 20.1°, 20.8°, There are characteristic peaks at 21.8° and 23.3°, which are confirmed to be Sofosbuvir Form 6.
Embodiment 2
[0025] Dissolve 10 grams of sofosbuvir in 80 milliliters of ethanol, slowly drop the solution into 480 milliliters of pure water preheated to 60° C., and add 0.05 grams of sofosbuvir crystal form 6 as a seed crystal. After the dropwise addition was completed, heat and stir for about 3 to 4 hours to crystallize, then lower the temperature to 0°C and stir for 2 to 3 hours, filter, and vacuum-dry the filter cake at 60°C to constant weight to obtain 9.6 grams of white solid with a yield of 96%. The purity is 99.6%. The X-ray powder diffraction pattern of the prepared product is consistent with the sofosbuvir crystal form 6.
Embodiment 3
[0027] Dissolve 10 grams of sofosbuvir in 60 milliliters of acetone, slowly add the solution dropwise to 480 milliliters of pure water preheated to 60° C., and add 0.2 grams of sofosbuvir crystal form 6 as a seed crystal. After the dropwise addition was completed, heat and stir for about 3 to 4 hours to crystallize, then lower the temperature to 0°C and stir for 2 to 3 hours, filter, and vacuum-dry the filter cake at 60°C to constant weight to obtain 9.4 grams of white solid with a yield of 94%. The purity is 99.5%. The X-ray powder diffraction pattern of the prepared product is consistent with the sofosbuvir crystal form 6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com