Sofosbuvir monocrystal M and preparation method and applications of sofosbuvir monocrystal M
A sofosbuvir single crystal technology, which is applied in the field of sofosbuvir single crystal M and its preparation and application, can solve the problems of curative effect, change of drug bioavailability, drug absorption, transport influence and other problems, and achieve good application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation of sofosbuvir single crystal
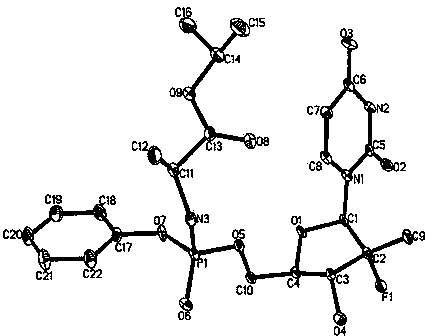
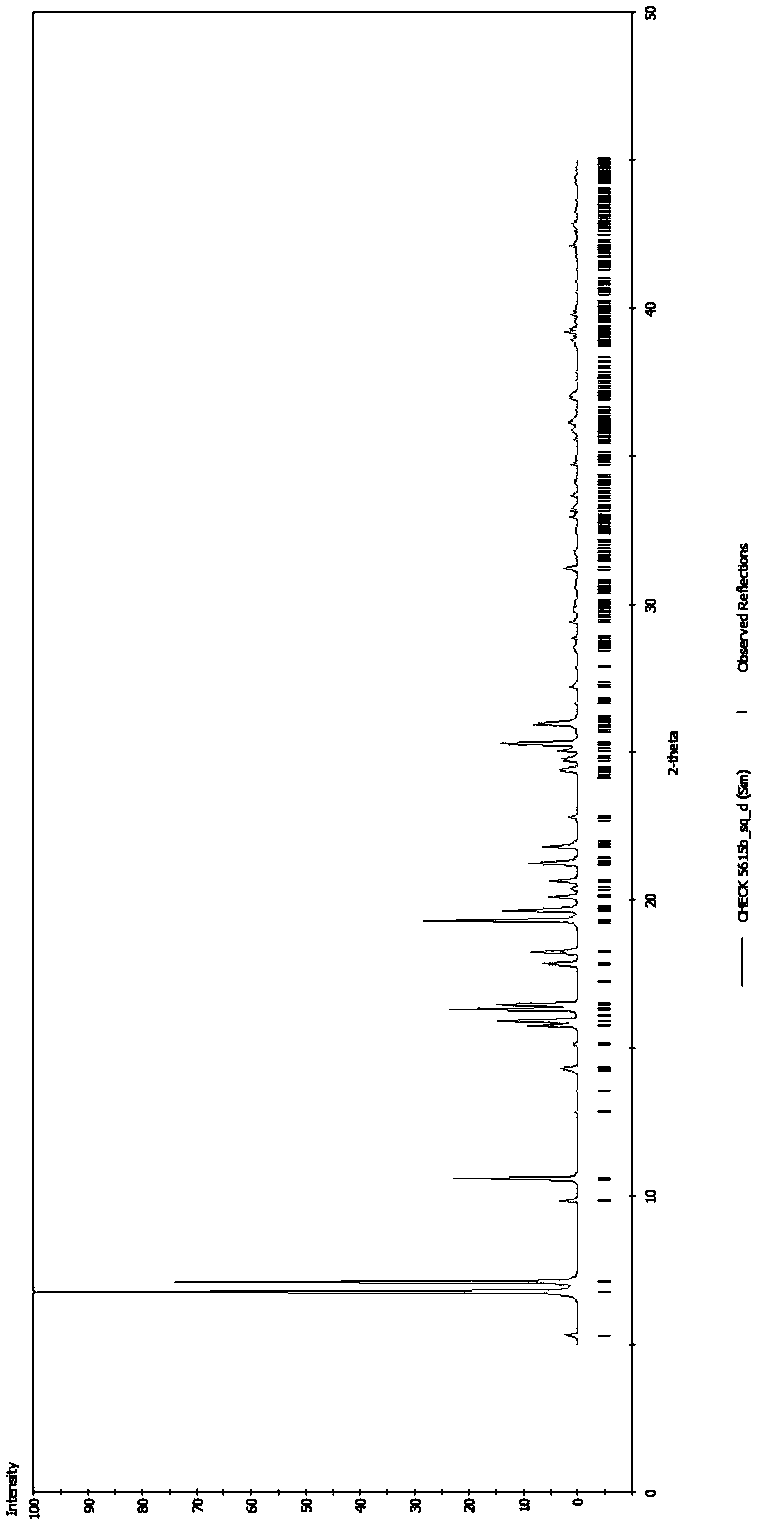
[0040] Dissolve 1g of Sofosbuvir in 20mL of 50°C chloroform-isopropanol (volume ratio 50:1) mixed solution, stir to dissolve, then cool the solution in which Sofosbuvir is dissolved to room temperature while stirring and crystallizing, Filter until no solid precipitates out, wash with a small amount of cold solvent (hexane or dichloromethane) (4°C), and send the sample for single crystal X-ray diffraction to determine the crystal structure. The obtained sofosbuvir is detected by HPLC, and the content is 99.9%; by gas chromatography, the residual solvent content is lower than 40ppm.
[0041] Its single crystal X-ray diffraction pattern has the following characteristics, the crystal belongs to the monoclinic system, the space group is P21, and the unit cell parameters: β = 108.96(3)°. unit cell volume The number of molecules in the unit cell is Z=2.
[0042] The drug used in the present invention is Sofosbuvir...
Embodiment 2
[0044] Embodiment 2: Preparation of Sofosbuvir single crystal
[0045] Dissolve 1g of Sofosbuvir in 6mL of dimethyl carbonate at 50°C, slowly crystallize under stirring, collect the crystals by filtration, wash with a small amount of cold solvent (hexane or dichloromethane), select the crystals and send them to the sample for determination Crystal X-ray diffraction to determine the three-dimensional structure of the obtained new crystal form of Sofosbuvir.
[0046] Its single crystal X-ray diffraction pattern has the following characteristics, the crystal belongs to the monoclinic system, the space group is P21, and the unit cell parameters: β = 108.96(3)°. unit cell volume The number of molecules in the unit cell is Z=2.
[0047] The drug used in the present invention is Sofosbuvir, and its chemical name is S-2-(((S)-(((2R,3R,4R,5R)-5-(2,4-oxo-3, 4-Dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyl-tetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propionic aci...
Embodiment 3
[0048] Embodiment 3: Preparation of new crystal form of Sofosbuvir
[0049] Dissolve 1 g of sofosbuvir in 10 mL of diethyl oxalate at 50°C, slowly cool to room temperature, and volatilize and crystallize. After no more solids were precipitated, the crystals were collected by filtration, washed with a small amount of cold solvent (hexane or dichloromethane), and then sent to determine single crystal X-ray diffraction.
[0050] Its single crystal X-ray diffraction pattern has the following characteristics, the crystal belongs to the monoclinic system, the space group is P21, and the unit cell parameters: β = 108.96(3)°. unit cell volume The number of molecules in the unit cell is Z=2.
[0051] The drug used in the present invention is Sofosbuvir, and its chemical name is S-2-(((S)-(((2R,3R,4R,5R)-5-(2,4-oxo-3, 4-Dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyl-tetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propionic acid iso Propyl ester, molecular formula C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com