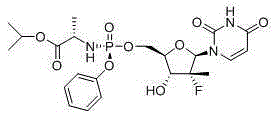
Preparation for sofosbuvir key intermediate
A -2-C-, methyl technology, applied in the field of preparation of key intermediates of sofosbuvir, can solve the problems of dark color of wastewater, long route, excessive oxidation and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example
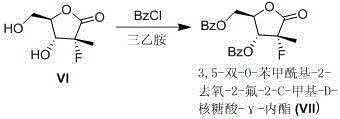
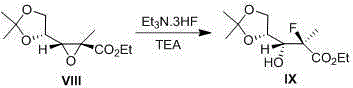
[0019] 1. Reaction formula
[0020]
[0021] 250mL four-necked flask, under magnetic stirring, add ethanol (60mL) and benzonitrile (4mL), start stirring and add potassium carbonate (8.1g, 58.6mol), the system temperature is cooled with ice water, and then slowly add 35%H 2 O 2 (76mL). then slowly add II (8.5g, 39.7mmol) in ethanol (40mL) was added to control the reaction temperature below 0°C. After the dropwise addition was complete, stir at a temperature below 0 °C until TLC showed the disappearance of the starting material. Water (30 mL) was added to the reaction system to quench the reaction, and ethyl acetate (100 mL) was added to the system to stir, and the layers were separated after standing, and the organic phase was separated. The aqueous phase was further extracted with ethyl acetate (2 x 50 mL). The organic phases were combined, and the organic phases were sequentially washed with 5% Na 2 SO 3 It was washed with aqueous solution (50 mL) and saturated br...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com