Crystal forms of sofosbuvir and preparation method of crystal forms
A sofosbuvir and crystal form technology, applied in the field of sofosbuvir crystal form and its preparation, can solve the problems of low solubility, low stability of sofosbuvir, poor repeatability of production process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] In the preparation method of the four crystal forms announced by the present invention, sofosbuvir is prepared according to the method reported in the existing literature (J.Med.Chem.2010,53:7202-7218); other All solvents and reagents were commercially available chemically pure or analytically pure products.
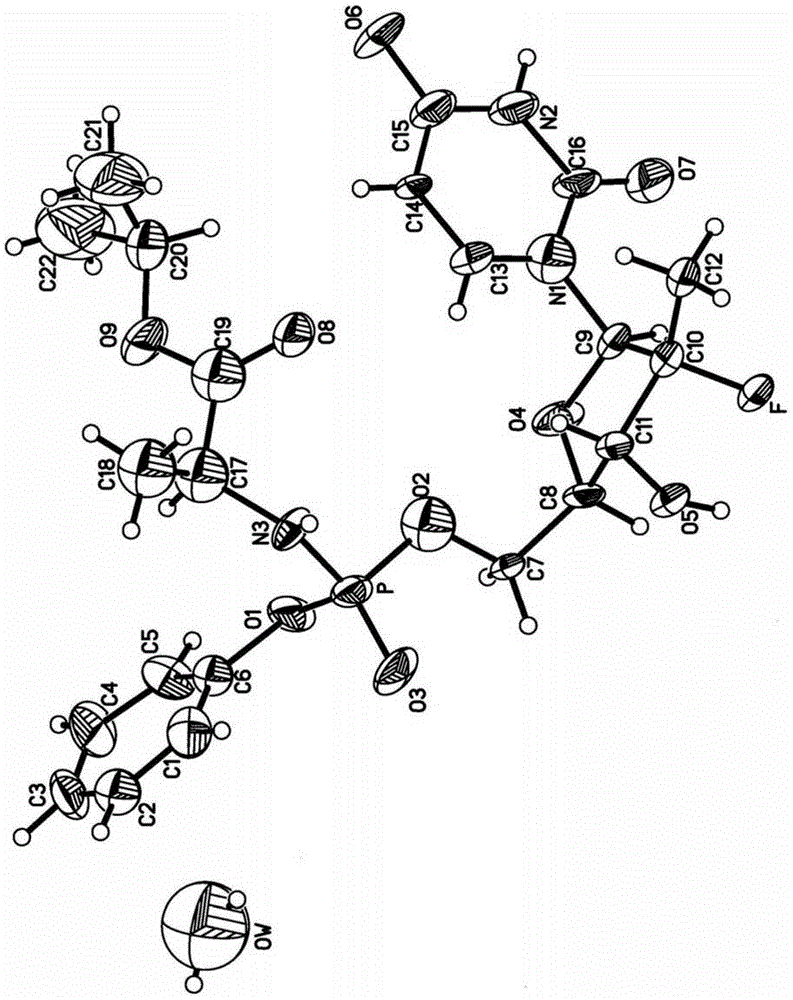
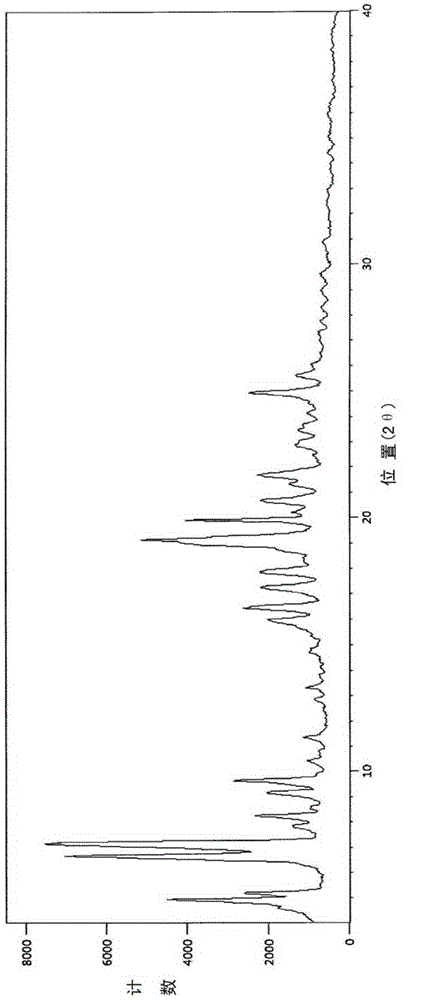
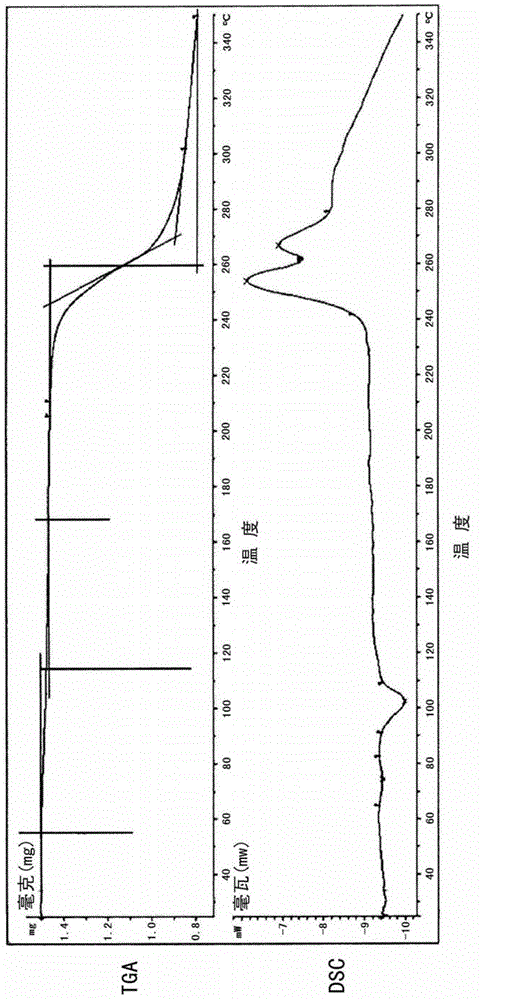
[0053] The four novel crystal forms disclosed in the present invention have been tested and characterized by single crystal X-ray diffraction structure analysis. Single crystal X-ray diffraction structure analysis can directly obtain the unit cell parameters, space group, number of molecules in the unit cell, solvent in the unit cell and the three-dimensional structure information of the molecules. It is the most direct, accurate and effective crystallographic research method at present. type analysis method.
[0054] The crystal structure determination instrument used in the embodiment of the present invention is a CAD4 / PC single crystal X-ray diffractometer of ...
Embodiment 1
[0064] Example 1: Preparation of Form H1 Single Crystal
[0065] Add 0.5 g of Sofosbuvir and 15 mg of water to a mixed solvent of 2.5 ml of anisole and 2.5 ml of dibutyl ether, heat the mixture to 80-85°C and stir to dissolve the solid completely, then 1-3°C / min The speed was slowly cooled to room temperature and stood at room temperature for 72 hours, and the precipitated colorless needle-shaped crystal form H1 single crystal was taken out by filtration.
[0066] Optionally, the mixed solvent in the above preparation method can be replaced with a mixed solvent of 2.5 ml of m-xylylene dimethyl ether and 2.5 ml of isopropyl ether.
[0067] Determination of the X-ray structure of the crystal form H1 single crystal in Example 1
[0068] Select the sofosbuvir crystal form H1 single crystal with a size of 0.30mm × 0.20mm × 0.10mm in the crystal obtained in Example 1, place it on a single crystal X-ray diffractometer, and collect it within the range of 1.20°≤2θ≤25.42° 5532 diffrac...
Embodiment 2
[0081] Embodiment 2: Preparation of crystal form H2 single crystal
[0082] Add 0.5 g of sofosbuvir and 0.05 g of methanol to 5.0 ml of m-phenylenedimethoxy ether solvent, heat to 85-90°C to dissolve the solid completely, then slowly cool to room temperature at a rate of 1-3°C / min and After standing still for 24-72 hours, the colorless needle-like crystal form H2 was taken out by filtration.
[0083] Optionally, the 5.0 ml m-xylylene solvent in the above preparation method can be replaced with a mixed solvent of 5.0 ml m-xylylene and 5.0 ml isopropyl ether.
[0084] Crystalline form H in embodiment 2 Determination of crystal structure
[0085] Select the sofosbuvir crystal form H2 single crystal with a size of 0.20mm × 0.10mm × 0.10mm in the crystal obtained in Example 2, place it on a single crystal X-ray diffractometer, and collect it within the range of 1.21°≤2θ≤25.40° 5446 diffraction data, including 5216 independent diffraction points. Collected data were corrected for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com