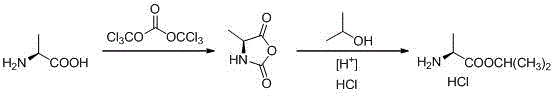
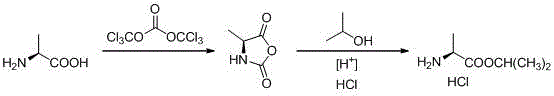
Novel preparation method of L-alanine isopropyl ester hydrochloride
A technology of isopropyl alanine and hydrochloride, which is applied in the preparation of organic compounds, chemical instruments and methods, and cyanide reaction preparation, etc., can solve the problems of high cost of waste treatment, ineffective separation, and increased difficulty in operation, etc. problem, to achieve the effect of reducing consumption, sufficient supply and improving utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A. Preparation of 4-methyl-2,5-diketooxazolidine (Ⅱ)
[0022] Add L-alanine (89g, 1.0mol), 1,2-dichloroethane (890g) into the reactor, stir well and then add solid phosgene (148g, 0.5mol) in batches under necessary cooling measures ), keep the temperature of the mixture not exceeding 60°C during the addition process, remove the cooling device after the addition, and keep the temperature at 60±5°C for 12 hours. Finally, add n-hexane (445g), stir for 1 hour, and collect the precipitated white or off-white solid by filtration, which is the crude product of 4-methyl-2,5-diketooxazolidine (II). After drying, 101.3g was obtained. The yield About 88.1%. The content detected by HPLC is greater than 95%, and the product can be directly used in the next reaction without further purification. After purification by sampling column chromatography, the detection spectrum data are as follows:
[0023] 1H NMR (CDCl3, 500MHz) δ: 1.53 (3H, d), 4.58-4.62 (H, m). FAB-MS (m / z): 116 (M+...
Embodiment 2
[0028] Other steps are the same as in Example 1, except that the preparation method of 4-methyl-2,5-diketooxazolidine (II) in step A is as follows:
[0029] Add L-alanine (89g, 1.0mol), 1,2-dichloroethane (180g) into the reactor, stir well and then add solid phosgene (99g, 0.33mol) in batches under necessary cooling measures ), keep the temperature of the mixture not exceeding 60°C during the addition process, remove the cooling device after the addition, and keep the temperature at 60±5°C for 6 hours. Finally, add n-hexane (445g), stir for 1 hour, and collect the precipitated white or off-white solid by filtration, which is the crude product of 4-methyl-2,5-diketooxazolidine (II). After drying, 83.5g was obtained. The yield About 72.6%. The content detected by HPLC is greater than 95%, and the product can be directly used in the next reaction without further purification. After purification by sampling column chromatography, the detection spectrum data are as follows:
[0...
Embodiment 3
[0032] Other steps are the same as in Example 1, except that the preparation method of 4-methyl-2,5-diketooxazolidine (II) in step A is as follows:
[0033] Add L-alanine (89g, 1.0mol), 1,2-dichloroethane (550g) into the reactor, stir well and then add solid phosgene (120g, 0.4mol) in batches under necessary cooling measures ), keep the temperature of the mixture not exceeding 60°C during the addition process, remove the cooling device after the addition, and keep the temperature at 60±5°C for 9 hours. Finally, add n-hexane (445g), stir for 1 hour, and collect the precipitated white or off-white solid by filtration, which is the crude product of 4-methyl-2,5-diketooxazolidine (II). After drying, 95.8g was obtained. The yield About 83.3%. The content detected by HPLC is greater than 95%, and the product can be directly used in the next reaction without further purification. After purification by sampling column chromatography, the detection spectrum data are as follows:
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com