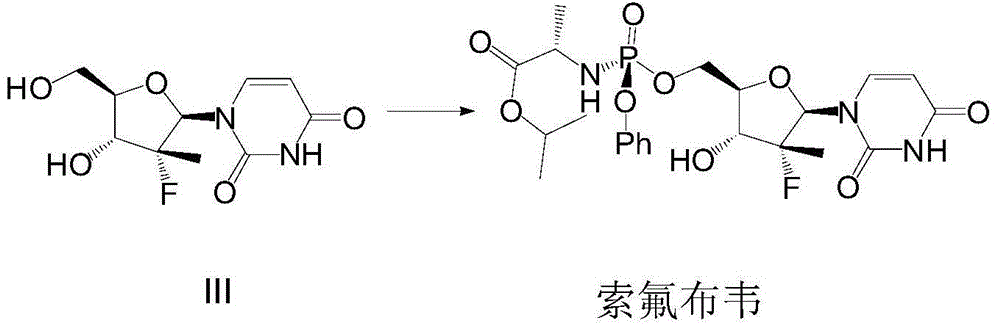
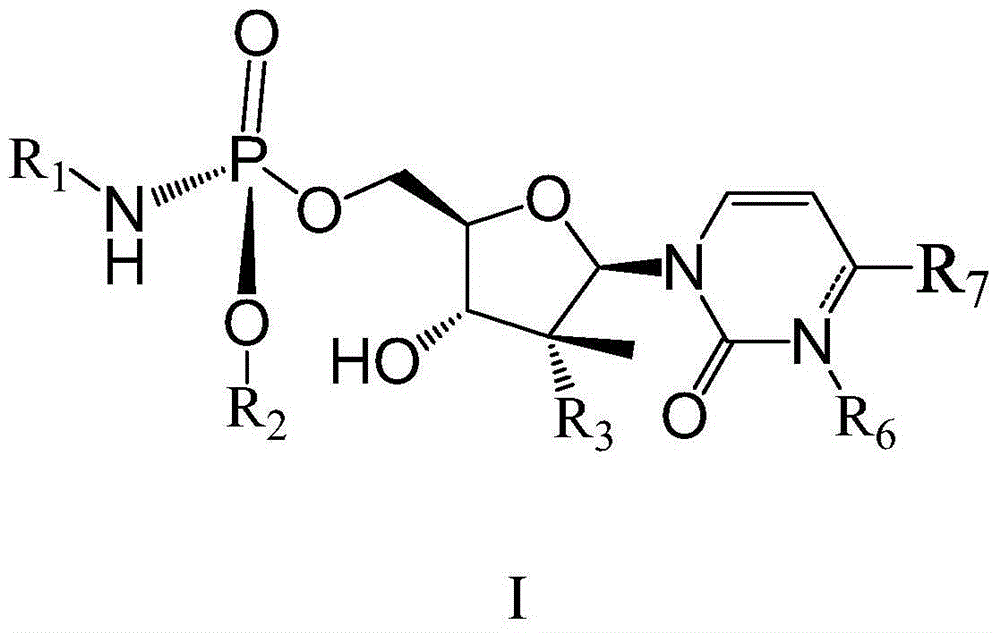
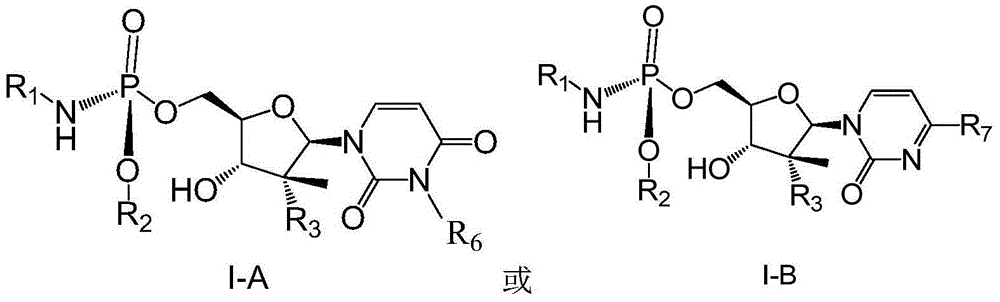
(2'R)-2'-deoxy-2'-halogenated-2'-methyluridine derivative, preparation method and uses thereof
A phenoxy, branched alkoxycarbonyl technology, applied in the field of preparing sofosbuvir and its analogs, can solve the problems of consumption of tert-butylmagnesium chloride, side reactions, difficulty in purification and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087]
[0088] Compound III-2 (0.260g, 1mmol) was dissolved in DMF (2ml), and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (0.30ml, 2mmol) was added , ice bath, after stirring for about 15 minutes, chloromethyl methyl ether (76 μl, 1mmol) was added, and after about 1 hour, TLC showed that the raw materials had basically reacted completely, and about 1ml of methanol was added to quench the reaction, and the compound II- 1 (200 mg).
[0089] 1 HNMR (300MHz, DMSO): 8.05(d, J=8.23Hz, 1H), 6.01(d, J=18.28Hz, 1H), 5.80(d, J=8.21Hz, 1H), 5.67(d, J=6.27 Hz,1H),5.31(t,J=5.72Hz,1H),5.17(s,2H),3.82(dt,J=6.32,13.43Hz,3H),3.63(d,J=10.81Hz,1H), 3.25(s,3H),1.25(d,J=22.45Hz,3H);ESI(M+Cl:339).
Embodiment 2
[0091] Compound II-1 (0.76g, 2mmol) was dissolved in THF (10mL), cooled to -5°C, added tert-butylmagnesium chloride (2.4mL, 2.4mmol), stirred for 30 minutes, raised to room temperature and stirred for 30 minutes, cooled To 5°C, add (S)-2-[(S)-(2,3,4,5,6-pentafluoro-phenoxy)-phenoxy-phosphoramido]propionic acid isopropyl ester (1.09 g, 2.4 mmol), after about 4 hours of reaction, TLC showed that the reaction was basically complete, and 917 mg of compound I-1 was obtained by column chromatography.
[0092] 1 HNMR (400M, DMSO): 7.64 (d, J = 8.19Hz, 1H), 7.38 (t, J = 7.89Hz, 2H), 7.21 (m, 3H), 6.06 (dd, J = 9.93, 12.96Hz, 2H ),5.88(d,J=7.03Hz,1H),5.70(d,J=8.15Hz,1H),5.18(s,2H),4.85(p,J=6.27Hz,1H),4.38(m,1H ), 4.25(dt, J=5.67, 12.02Hz, 1H), 4.03(dd, J=4.73, 10.05Hz, 1H), 3.81(m, 2H), 3.27(s, 3H), 1.24(m, 6H) ,1.15(d,J=6.26Hz,6H);ESI(M-1:572).
Embodiment 3
[0094] Compound I-1 (100mg, 0.17mmol) was dissolved in tetrahydrofuran (4ml), 1ml of 10% hydrochloric acid was added, heated for reaction, the reaction was complete after about 4 hours, and sofosbuvir (10mg) was obtained by column chromatography.
[0095] 1 HNMR (400M, DMSO): 11.52(s, 1H), 7.57(d, J=8.17Hz, 1H), 7.37(m, 2H), 7.22(m, 3H), 6.04(m, 2H), 5.86(d ,J=7.08Hz,1H),5.54(d,J=8.06Hz,1H),4.86(p,J=6.25Hz,1H),4.38(dd,J=5.82,11.71Hz),4.24(dt,J =5.77,11.74Hz,1H),4.01(m,1H),3.81(m,2H),1.25(m,6H),1.15(d,J=6.29Hz,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com